Phosphorus and Molybdenum Problems for Life on Other Worlds
Stymied by attempts to find life-friendly conditions on surfaces of extraterrestrial planets and moons, astrobiologists have turned to increasingly exotic sites. Two such sites are (1) the upper atmospheres of planets and moons with thick atmospheres and (2) possible pools of liquid water below the surfaces of planets and moons. A nearby example of the former are Venus’s high clouds. Nearby examples of the latter are Europa and Enceladus, moons of Jupiter and Saturn, respectively.
A recent paper published by astrophysicists Manasvi Lingam and Abraham Loeb in the Astronomical Journal casts doubt on the suitability for life in upper atmospheres and subsurface lakes or oceans.1 The paper explains how these sites are likely plagued with extreme shortages of phosphorus, molybdenum, and other life-essential elements.
What Life Requires
All conceivable carbon-based life-forms require the availability of both phosphorus and molybdenum. Phosphates are an essential component of DNA, RNA, ATP, and phospholipids. Molybdenum is essential for the functioning of several life-critical proteins2 and for animals it eliminates toxic reactions to sulfites in food.3
Four decades ago, biochemists discovered that any conceivable physical life-form must be carbon-based. Carbon is the only element in the periodic table that permits the chemical bonding diversity, complexity, and stability life molecules require.
Both phosphorus and molybdenum are relatively rare. Phosphorus comprises 1,050 parts per million by mass in Earth’s crust4 while molybdenum makes up just 1.2 parts per million.5 And yet Earth is extraordinarily rich in both of these elements. Compared to the rest of our galaxy and the universe Earth’s phosphorus to magnesium ratio is four times higher and its molybdenum to magnesium ratio is five times higher. Yet even with this great abundance of phosphorus and molybdenum, life productivity on Earth is limited by their availability.
Just-Right Phosphorus Abundances Needed
In their paper, Lingam and Loeb first cite research published a year ago6 that established that previous to 600 million years ago Earth’s abundance of oceanic phosphorus was less than a fifth of what it is today. What kept the phosphorus concentration so low was scavenging of phosphorus into ferrous minerals, absorption into iron oxides, and limited recycling in what was then an oxidant-poor ocean.
This paucity of phosphorus limited life in the oceans to microbes at an abundance and diversity level far below the present. What phosphorus existed in the oceans at that time came almost entirely from the runoff of rivers on the continental landmasses.
The Harvard astrophysicists then pointed out the obvious. Ice-covered worlds like Europa and Enceladus will not have any riverine input of phosphorus. Furthermore, the liquid water oceans that might exist below these worlds’ ice crusts very likely will have either a neutral or basic pH. A significant body of liquid water below a surface ice crust also implies the very high likelihood of hydrothermal activity at the bottom of the liquid water pool (see figure 1). Hydrothermal activity efficiently removes phosphorus from subterranean water pools.

Figure 1: Artist’s rendering of hydrothermal activity on the seafloor of Enceladus. Image credit: NASA/JPL
Lingam and Loeb conservatively calculated that the abundance of phosphorus in subterranean oceans of ice-covered worlds will be 1,000 to 100,000 times lower than in Earth’s oceans. Such an extremely low abundance of phosphorus makes the survival of life, let alone the origin of life, a near impossibility.
Other Just-Right Mineral Abundances Needed
The researchers go on to address the availability of other life-essential elements in ice-covered worlds. They note that the only significant source of bioavailable nitrogen in ice-covered worlds will be submarine weathering. Though they were not able to produce a precise estimate of the amount of bioavailable nitrogen produced by such weathering, they conclude that it would be much below “the corresponding value for continental weathering by rain water.”7
Next, Lingam and Loeb examine bioavailable iron. For Earth, virtually all the bioavailable iron in the oceans comes from eolian mineral dust, interplanetary dust, and subaerial continental weathering.8 None of these sources are available in subsurface ocean worlds. Thus, such worlds will lack not only the phosphorus abundance that life needs but also lack the nitrogen, iron, and possibly several other life-essential elements.
More Habitability Constraints
The pair of researchers did not address the problem of too much subsurface liquid water. If this water adds up to more than 1 percent of the mass fraction of a planet or large moon, the pressure from the water depth will form a thick, impenetrable ice layer on the ocean floor (see figure 2).9 For such worlds there will be no submarine weathering and, thus, no delivery of phosphorus, nitrogen, iron, copper, molybdenum, and other life-essential elements into the liquid water ocean.
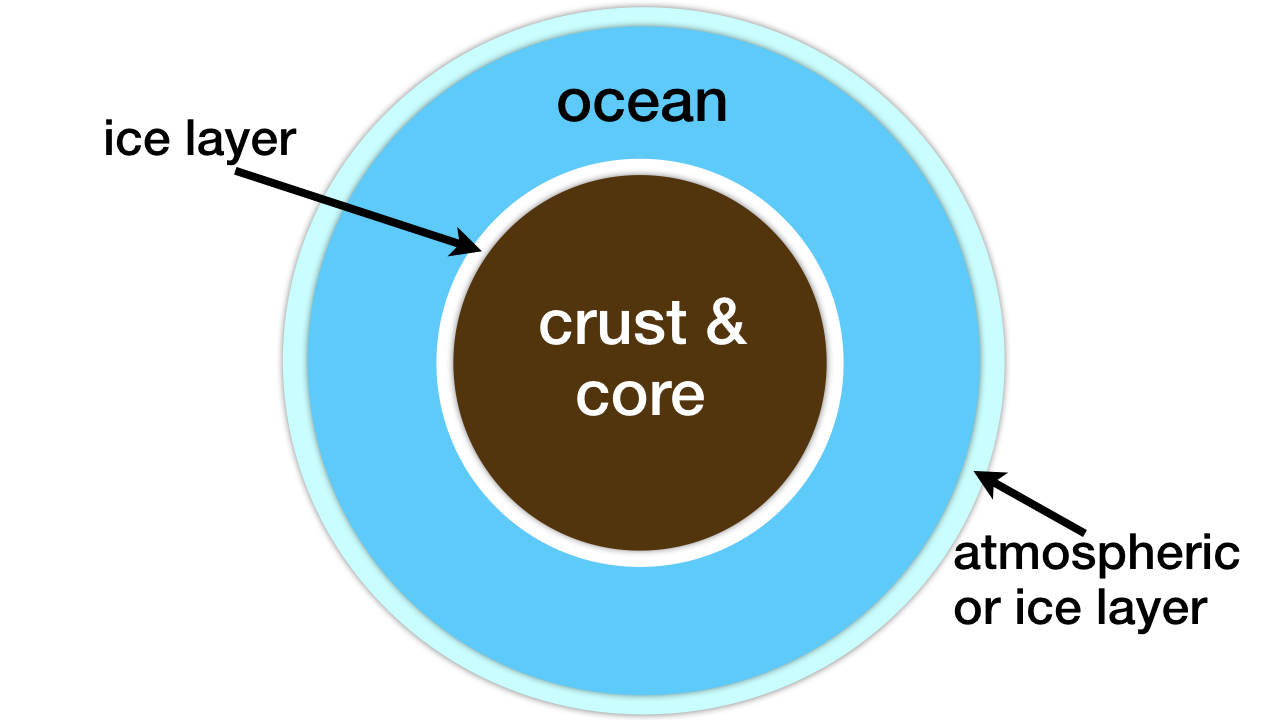
Figure 2: Deep ocean water worlds. The subsurface ice layer prevents the weathering of rock and, thus, the delivery of life-essential elements into the liquid water ocean. Image credit: Hugh Ross
Lingam and Loeb close their paper by considering the habitability of planets like Venus (see figure 3). For the past fifty years, astronomers have speculated that life could conceivably survive at those altitudes in the upper atmospheres of Venus-like planets where the temperatures would allow water to exist in the liquid state.10 However, neither the Galileo orbiter nor the Venus Express mission has found any evidence for molybdenum in Venus’s atmosphere.11 Without molybdenum and other bioessential trace elements life is not possible in the atmosphere of Venus or planets like it.
Figure 3: Venus and its thick clouds. Above Venus’s dense carbon dioxide layer is a thick layer of sulfuric acid. Image credit: NASA
The researchers do not rule out life in subsurface ocean worlds or Venus-like planets, but they conclude it is unlikely. However, given the degree to which the availability of phosphorus, nitrogen, iron, and molybdenum limits life on Earth, the case must be far more constraining for the exotic sites. The evidence Lingam and Loeb provide for all these nutrients being orders of magnitude less abundant in subsurface ocean worlds (and if the ocean is deep, nonexistent) and Venus-like planets than on Earth causes me to question whether life can survive on such worlds.
Earth’s Just-Right Abundance Levels
Scientists already know that plants and animals cannot survive on so little phosphorus, nitrogen, iron, and molybdenum. Laboratory experiments can settle whether there is any possibility for long-term survival of microbes.
In the meantime, these Harvard astrophysicists give us yet more reasons to be grateful that we live on such an improbable planet. Our planetary home has the most anomalous abundances of elements, especially when it comes to vital poisons. Earth’s crust contains just-right abundance levels of all twenty-two vital poison elements.12 Vital poisons are elements that if too abundant will kill life, but if too under-abundant will also kill life. So much “just-rightness” points to a supernatural Creator who has a special love for human beings.
Featured image: Molybdenum on the left and phosphorus on the right. Image credits: Alchemist-hp and YouTube, respectively.
Endnotes
- Manasvi Lingam and Abraham Loeb, “Is Extraterrestrial Life Suppressed on Subsurface Ocean Worlds Due to the Paucity of Bioessential Elements?” Astronomical Journal 156 (October 2018): id. 151, doi:10.3847/1538-3881/aada02.
- Ralf R. Mendel, “Cell Biology of Molybdenum,” BioFactors 35 (September/October 2009): 429–34, doi:10.1002/biof.55.
- Harvey J. Cohen et al., “Molecular Basis of the Biological Function of Molybdenum: The Relationship Between Sulfite Oxidase and the Acute Toxicity of Bisulfite and SO2,” Proceedings of the National Academy of Sciences USA 70 (December 1973): 3655–59, doi:10.1073/pnas.70.12.3655.
- “The Element Phosphorus,” Thomas Jefferson National Accelerator Facility – Office of Science Education, accessed November 15, 2018, https://education.jlab.org/itselemental/ele015.html.
- “The Element Molybdenum,” Thomas Jefferson National Accelerator Facility – Office of Science Education, accessed November 15, 2018, https://education.jlab.org/itselemental/ele042.html.
- Michael A. Kipp and Eva E. Stüeken, “Biomass Recycling and Earth’s Early Phosphorus Cycle,” Science Advances 3 (November 22, 2017): id. eaao4795, doi:10.1126/sciadv.aao4795; Toby Tyrrell, “The Relative Influences of Nitrogen and Phosphorus on Oceanic Primary Production,” Nature 400 (August 5, 1999): 525–31, doi:10.1038/22941.
- Lingam and Loeb, “Is Extraterrestrial Life Suppressed?”, 5.
- Lingam and Loeb, 5.
- A. Levi, D. Sasselov, and M. Podolak, “The Abundance of Atmospheric CO2 in Ocean Exoplanets: A Novel CO2 Deposition Mechanism,” Astrophysical Journal 838 (March 20, 2017): id. 24, doi:10.3847/1538-4357/aa5cfe.
- Harold Morowitz and Carl Sagan, “Life in the Clouds of Venus?”, Nature 215 (September 16, 1967): 1259–60, doi:10.1038/2151259a0; Dirk Schulze-Makuch et al., “A Sulfur-Based Survival Strategy for Putative Phototrophic Life in the Venusian Atmosphere,” Astrobiology 4 (March 2004): 11–18, doi:10.1089/153110704773600203; Sanjay S. Limaye et al., “Venus’ Spectral Signatures and the Potential for Life in the Clouds,” Astrobiology 18 (September 2018): 1181–98, doi:10.1089/ast.2017.1783.
- Emmanuel Marcq et al., “Composition and Chemistry of the Neutral Atmosphere of Venus,” Space Science Reviews 214 (February 2018): id. 10, doi:10.1007/s11214-017-0438-5.
- Hugh Ross, Improbable Planet: How Earth Became Humanity’s Home (Grand Rapids: Baker, 2016), 166–68.
Check out more from Dr. Hugh Ross @Reasons.org





