Origin of Our Amazing Nitrogen
For several decades, astronomers have known that Earth’s atmosphere possesses a nitrogen abundance that is exquisitely fine-tuned for advanced life. Now, a team of four astronomers has discovered the origin of Earth’s atmospheric nitrogen.
Nitrogen dominates Earth’s atmosphere. By volume, it comprises 78.084 percent of Earth’s atmosphere. Earth is the only known planetary body where nitrogen is the dominant atmospheric component (see figure). The only other known body where nitrogen dominates is in the stratosphere of Saturn’s moon Titan. Nitrogen comprises 98.4 percent of the atmospheric layer 40–300 kilometers above Titan’s surface.
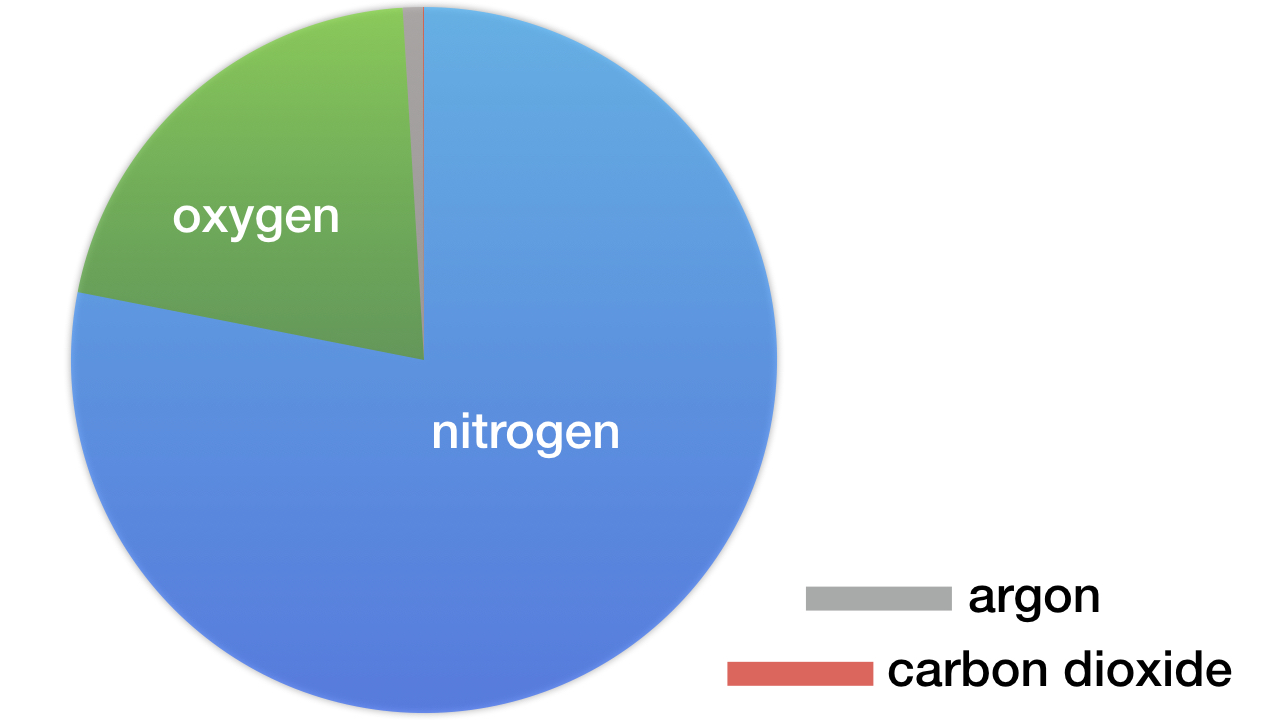
Figure: Earth’s Atmospheric Composition. By volume, dry air contains 78.084 percent nitrogen, 20.946 percent oxygen, 0.93 percent argon, 0.04 percent carbon dioxide, and trace amounts of several other gases. Depending on altitude and weather conditions, water vapor can comprise from 0.0 to 1.2 percent of the atmosphere. Diagram credit: Hugh Ross
By contrast, nitrogen is rare in Earth’s crust. It only makes up 0.0019 percent (19 parts per million). This rarity is consistent with Earth’s overall nitrogen abundance. Earth’s nitrogen-to-iron ratio is about 2,400 less than that for the rest of the Milky Way Galaxy.
Sources of Earth’s Nitrogen
As I described in some detail in my book Improbable Planet, Earth lost virtually all its nitrogen through two events early in its history. The first was nearby supernovae and Wolf-Rayet starsblasting the primordial solar system with aluminum-26 and other short-lived radioisotopes that evaporated away nearly all the gases and liquids from the emerging solar system bodies.1 The second occurred about 95 million years later: the merger between proto-Earth and Theia (another solar system planet) that led to the formation of the Moon and the elimination of nearly all that remained of Earth’s atmosphere and ocean.2
Four astronomers, led by University of Nevada, Las Vegas’s Cheng Chen, had a paperpublished in the February 2019 issue of the Astronomical Journal in which they demonstrated that Earth very likely received a late delivery of nitrogen that added to the nitrogen that survived the two events described above.3 Chen et al. also determined where this late delivery came from.
Chen’s team first cited recent nitrogen isotope ratio measurements of (1) Earth’s present-day atmosphere, (2) Earth’s mantle, and (3) Earth’s Archean sedimentary rocks and crustal hydrothermal systems. For Earth’s present-day atmosphere, the nitrogen-15 to nitrogen-14 ratio = 0.003676. For Earth’s mantle, nitrogen-15/nitrogen-14 ranges from 0.003566 to 0.003658. Records of Archean sedimentary rocks and crustal hydrothermal systems, circa 4–2.5 billion years ago, implied that nitrogen-15/nitrogen-14 in Earth’s Archean atmosphere was 0.003786. However, less metamorphosed Archean sedimentary rocks and trapped Archean seawater indicated nitrogen-15/nitrogen-14 in Earth’s Archean atmosphere was only slightly above that of Earth’s present-day atmosphere.
Nitrogen-15/nitrogen-14 in Earth’s mantle indicates Earth’s nitrogen status after the Moon-forming event. That Earth’s present-day atmosphere has a higher nitrogen-15/nitrogen-14 ratio than Earth’s mantle shows that Earth’s atmosphere received a late delivery of nitrogen-15 enriched gas. That Archean records reveal Earth’s Archean atmosphere had an even higher nitrogen-15/nitrogen-14 ratio than Earth’s present atmosphere indicates the timing for the late delivery of nitrogen-15 enriched gas.
It is well established that cold, dense gas where carbon monoxide is frozen out generates nitrogen-15 enriched gas. Such cold, dense gas exists in the solar system’s Kuiper Belt, the comet belt existing beyond the orbit of Neptune. Indeed, some Kuiper Belt comets have measured nitrogen-15/nitrogen-14 rations about twice that of Earth’s mantle.4 Thus, Chen et al. concluded that 5–10 percent of Earth’s atmospheric nitrogen came to Earth from Kuiper Belt comets sometime during the Archean era.
Atmospheric Nitrogen Fine-Tuning
The extra nitrogen delivered to Earth’s atmosphere made possible both the long-term survival of primitive life and the relatively short-term survival of advanced life. If the nitrogen content in Earth’s atmosphere were any less, Earth would have lost its surface water to outer space.
Nitrogen is not a greenhouse gas, but its presence in the atmosphere enhances the greenhouse heat-trapping capability of both carbon dioxide and methane. If the quantity of nitrogen in Earth’s atmosphere were any greater, Earth’s surface temperature would be too hot for advanced life. If the quantity of nitrogen in Earth’s atmosphere were any lower, Earth’s surface temperature would be too cold for advanced life to survive.
The ratio of molecular nitrogen to molecular oxygen determines whether lungs can efficiently function for many continuous years. The nitrogen buffers the oxygen. If the oxygen-to-nitrogen ratio in Earth’s atmosphere were even very slightly lower or very slightly higher than what it is, birds and mammals would not be able to sustain high activity levels for years on end.
The quantity of nitrogen in Earth’s atmosphere determines the degree of nitrogen fixation that occurs. Less atmospheric nitrogen or more atmospheric nitrogen than what is now occurring would change the level of nitrogen fixation and lower the diversity of plant species.
The bottom line: there are at least four different reasons why the amount of nitrogen in Earth’s atmosphere must be fine-tuned. This fine-tuning implies that both the primordial terrestrial nitrogen and the later delivery of nitrogen to Earth must be fine-tuned. Such fine-tuning is indicative of the supernatural handiwork of the Creator.
Check out more from Reasons to Believe @Reasons.org
Endnotes
- Hugh Ross, Improbable Planet: How Earth Became Humanity’s Home (Grand Rapids, MI: Baker, 2016), 37–39.
- Ross, Improbable Planet, 48–57.
- Cheng Chen et al., “Late Delivery of Nitrogen to the Earth,” Astronomical Journal 157, no. 2 (February 2019): id. 80, doi:10.3847/1538-3881/aaf96a.
- J. Manfroid et al., “The CN Isotopic Ratios in Comets,” Astronomy & Astrophysics 503, no. 2 (August 4, 2009): 613–24, doi:10.1051/0004-6361/200911859.





