Did Ancient Carbon Dioxide Compensate for a Cooler Sun?
Most of us have only scant memories of our early childhood. While I can recall many details from when I was four years old, I have only a few memories from my third year, such as the layout and furnishings of the small apartment that my parents, my two sisters, and I lived in not far from McGill University in Montreal. I also remember a couple of walks in Montreal with my parents. As for my second year, I have only one intact memory—that of seeing the outside and inside of a wooden station wagon belonging to the hydraulics engineering company my father founded. I wish I had more detailed and extensive memories, but I do not.
Scientists face the same dilemma about Earth’s infancy. They possess a few fuzzy snapshots of the conditions existing on the early Earth, but they desire a much more comprehensive and detailed picture. Thanks to a new discovery,1 their desire is beginning to be fulfilled, and that fulfillment reveals more evidence for the fine-tuning of the early Earth in preparation for human beings and human civilization.
It is now established that life existed on Earth’s surface as far back as 3.825 billion years ago.2 However, it is also well established that the Sun at that time was 18–23 percent dimmer than it is now.3 Life can tolerate only about a 1–2 percent change in solar luminosity. For life to have survived on the surface of the early Earth, there must have been a much greater quantity of greenhouse gases in the atmosphere.
Scientists look to fossil and isotope evidence, which indicates what kinds and abundances of life were present on the early Earth, for clues. Based on that evidence they have deduced roughly what quantities of the greenhouse gases—carbon dioxide, methane, and water vapor—and what quantity of the greenhouse gas enhancer, nitrogen, likely existed at different times in early Earth’s atmosphere. What these scientists lacked, though, were direct measurements of the atmospheric abundances of these gases. A recently published report now addresses this lack.
Ancient Micrometeorites
Estimates for the atmospheric carbon dioxide levels from 3.8–2.5 billion years ago were based on paleosols (soils preserved by burial underneath sediments or volcanic deposits) and other proxies. These estimates ranged from about 0.003 to 0.75 bar of carbon dioxide. (1 bar = 100,000 pascals.4 Present mean atmospheric pressure on Earth at sea level = 1.01325 bar).
The carbon dioxide level in Earth’s atmosphere is not the only uncertainty that had puzzled scientists about conditions on the early Earth. There also exists a temperature uncertainty.
Isotopes of phosphates5 and deuterium6 suggest that Earth’s surface temperature during the Archean Eon (3.8–2.5 billion years ago) was less than 40°C. However, isotopes of oxygen in Archean cherts7 indicate that Earth’s surface temperature was about 70°C. Meanwhile, the presence of Archean glacial deposits8 shows that, at least on brief occasions during the Archean Eon, parts of Earth were less than 0°C.
These uncertainties motivated an interdisciplinary team of five scientists at the University of Washington (UW) to search for a more reliable and accurate proxy for Archean atmospheric carbon dioxide levels and for Archean surface temperatures. The proxy they chose was to determine the degree of oxidation in Archean micrometeorites caused by exposure to atmospheric carbon dioxide.
Iron-nickel metallic micrometeoroids, when they enter Earth’s atmosphere at high velocities, will briefly melt.9 While molten, the oxygen or carbon dioxide in the atmosphere can oxidize some or all of the iron metal. Before reaching Earth’s lower atmosphere, the molten micrometeoroids solidify. The solidified micrometeorites remain inert and thus preserve their oxidation state at the time they entered Earth throughout geologic time.10
Determination of Atmospheric CO2 Levels
There is no doubt that molecular oxygen (O2) is a much more efficient oxidizer of iron than is carbon dioxide (CO2). However, measurements establish that molecular oxygen remained at an extremely low abundance level in Earth’s atmosphere throughout the Archean Eon, only about 1 part per million by volume.11 Analysis of the acid weathering of Archean soils that became paleosols12 and Archean carbon cycle models13 show that the Archean atmosphere was very rich in carbon dioxide. Consequently, the five UW scientists concluded that the oxidation of Archean micrometeorites was entirely due to their exposure to carbon dioxide in Earth’s upper atmosphere.
The UW team measured the degree of oxidation in a sample of Archean micrometeorites dated at 2.7 billion years ago. Their analysis showed that 2.7 billion years ago, carbon dioxide comprised at least 70 percent by volume of Earth’s atmosphere.14 That is, assuming the Archean barometric air pressure was the same as it is today, carbon dioxide in Earth’s atmosphere 2.7 billion years ago was at least 1,750 times more abundant than it is today and at least 2,550 times as abundant as it was at the beginning of the industrial revolution.
The assumption that the Archean barometric air pressure was the same as it is now has been proven incorrect. Two studies, one based on gas bubbles in basaltic lava flows that solidified at sea level about 2.7 billion years ago in the Pilbara Craton, Australia,15 and the other based on fossilized raindrop splash patterns in volcanic tuffs of the Ventersdorp Supergroup, South Africa, also dated at 2.7 billion years ago,16 show that the barometric air pressure 2.7 billion years ago was only 0.25–0.50 of what it is today. Thus, the quantity of carbon dioxide in Earth’s atmosphere 2.7 billion years ago was 640–1,270 times what it was at the beginning of the industrial revolution.
Faint Sun Paradox Resolution
The five scientists concluded their paper with a calculation of the global mean temperature 2.7 billion years ago. Assuming the Sun was 20 percent dimmer and the air pressure half of what it is today, their determination that carbon dioxide comprised at least 70 percent by volume of Earth’s atmosphere yielded a global mean temperature of 30°C. If the air pressure were only one quarter of what it is today, the global mean temperature would be close to what it is today, namely 17°C. Hence, the researchers wrote, “Even if the early atmosphere was thinner than today, the elevated CO2 level indicated by our model result would help resolve how the Late Archean Earth remained warm when the young Sun was ~20% fainter.”17
The figure below shows the luminosity history of the Sun. In my book Improbable Planet, I devoted an entire chapter to the Sun’s past dimmer history—what is known as the faint Sun paradox.18 There, I described six major factors that in combination compensated for the dimmer Sun so that life could thrive during the Archean Eon:
- greater amounts of carbon dioxide and methane in Earth’s atmosphere
- greater volcanic release of greenhouse gases
- less continental landmass coverage of Earth’s surface
- a more rapid rotation rate
- a lower cosmic ray flux
- a slightly more massive young Sun
I also described several other factors that played minor roles in compensating for the dimmer Sun.
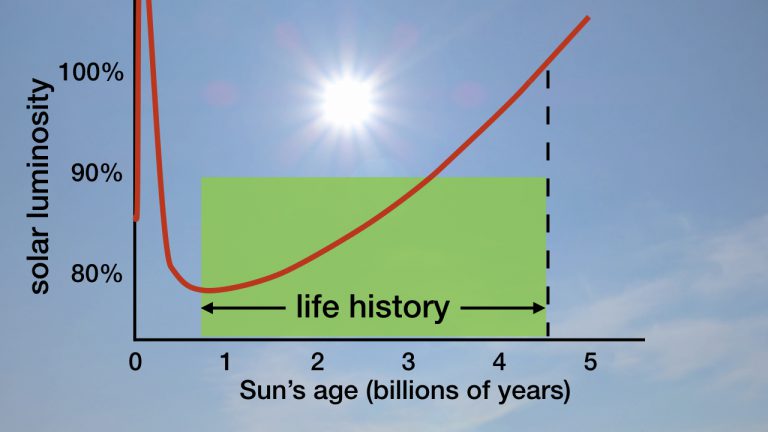
Figure: Sun’s Luminosity History Relative to Its Present Brightness. Credit: Hugh Ross
The research team’s measurements and analysis show that a very high abundance level of carbon dioxide in Earth’s Archean atmosphere played the most significant role in compensating for the dimmer Sun. So much so that scientists no longer need to look for additional missing pieces to explain how life could thrive on Earth with the Sun being about 20 percent dimmer than it is now. The faint Sun paradox is resolved.
The paradox has become resolvable by what we know and understand about the conditions on Earth during the Archean Eon. But it does not mean there is nothing more to learn about Earth’s design features that have made possible abundant, diverse life throughout the past 3.8 billion years—features that also opened up a brief window of time in which humans can live and thrive.
Scientists know that the atmospheric carbon dioxide level gradually declined throughout the past 3.8 billion years.19 However, they lack accurate measures of this decline from 3.8–0.6 billion years ago. As the UW scientists pointed out, the next step is to analyze iron-rich micrometeorites with ages spanning from the beginning of the Archean to the end of the Neoproterozoic Eons (from 3.8–0.6 billion years ago).
Measuring the fractional area of unoxidized iron in these iron-rich micrometeorites will tell us the quantity of carbon dioxide in Earth’s atmosphere at the measured dates of the different micrometeorites (with the exception of the brief episode known as the Great Oxygenation Event that took place 2.45–2.20 billion years ago). The possession of accurate measures of the atmospheric carbon dioxide levels from 3.80–2.45 and 2.20–0.58 billion years ago will yield a much more detailed and extensive picture of all the amazing fine-tuned designs that perfectly compensated for the Sun’s ongoing brightening so that abundant, diverse life could exist on Earth throughout the past 3.8 billion years.
Featured image: Artist’s Conception of the Archean Eon. Image credit: Tim Bertelink, Creative Commons Attribution
Check out more from Reasons to Believe @Reasons.org
Endnotes
- O. R. Lehmer et al., “Atmospheric CO2 Levels from 2.7 Billion Years Ago Inferred from Micrometeorite Oxidation,” Science Advances 6, no. 4 (January 22, 2020): eaay4644, doi:10.1126/sciadv.aay4644.
- Craig E. Manning, Stephen J. Mojzsis, and T. Mark Harrison, “Geology, Age and Origin of Supracrustal Rocks at Akilia, West Greenland,” American Journal of Science 306, no. 5 (May 2006): 303–66, doi:10.2475/05.2006.02 ; Kevin D. McKeegan, Anatoliy B. Kudryavtsev, and J. William Schopf, “Raman and Ion Microscopic Imagery of Graphitic Inclusions in Apatite from Older Than 3830 Ma Akilia Supracrustal Rocks, West Greenland,” Geology 35, no. 7 (July 2007): 591–94, doi:10.1130/G23465A.1; Allen P. Nutman and Clark R. L. Friend, “Raman and Ion Microscopic Imagery of Graphitic Inclusions in Apatite from Older Than 3830 Ma Akilia Supracrustal Rocks, West Greenland: Comment,” Geology 35, no. 1 (January 2007): e169, doi:10.1130/G24384C.1; Kevin D. McKeegan, Anatoliy B. Kudryavtsev, and J. William Schopf, “Raman and Ion Microscopic Imagery of Graphitic Inclusions in Apatite from Older Than 3830 Ma Akilia Supracrustal Rocks, West Greenland: Comment and Reply: Reply,” Geology 35, no. 1 (January 2007): e170, doi:10.1130/G24987Y.1; Yoko Ohtomo et al., “Evidence for Biogenic Graphite in Early Archaean Isua Metasedimentary Rocks,” Nature Geoscience 7 (January 2014): 25–28, doi:10.1038/ngeo2025; N. H. Sleep, E. Pope, and D. Bird, “Two-Way Feedback between Biology and Deep Earth Processes,” Abstract (American Geophysical Union, Fall Meeting 2012), abstract id.P14A-07; N. H. Sleep, “Tectonics and the Photosynthetic Habitable Zone,” Abstract (American Geophysical Union, Fall Meeting 2009), abstract id.B11E-03; N. H. Sleep and D. K. Bird, “Biological Modulation of Tectonics,” Abstract (American Geophysical Union , Fall Meeting 2008), abstract id.U42B-04.
- Hugh Ross, Improbable Planet: How Earth Became Humanity’s Home (Grand Rapids, MI: Baker, 2016).
- David C. Catling and James F. Kasting, Atmospheric Evolution on Inhabited and Lifeless Worlds (New York: Cambridge University Press, 2017).
- Ruth E. Blake, Sae Jung Chang, and Aivo Lepland, “Phosphate Oxygen Isotopic Evidence for a Temperate and Biologically Active Archaean Ocean,” Nature 464 (April 15, 2010): 1029–32, doi:10.1038/nature08952.
- M. T. Hren, M. M. Tice, and C. P. Chamberlain, “Oxygen and Hydrogen Isotope Evidence for a Temperate Climate 3.42 Billion Years Ago,” Nature 462 (November 12, 2009): 205–208, doi:10.1038/nature08518.
- L. Paul Knauth and Donald R. Lowe, “High Archean Climatic Temperature Inferred from Oxygen Isotope Geochemistry of Cherts in the 3.5 Ga Swaziland Supergroup, South Africa,” Geological Society of America Bulletin 115, no. 5 (May 2003): 566–80, doi:10.1130/0016-7606(2003)115<0566:HACTIF>2.0.CO;2.
- Maarten J. de Wit and Harald Furnes, “3.5-Ga Hydrothermal Fields and Diamictites in the Barberton Greenstone Belt—Paleoarchean Crust in Cold Environments,” Science Advances 2, no. 2 (February 26, 2016): e1500368, doi:10.1126/sciadv.1500368.
- Matthew J. Genge, “The Origins of I-Type Spherules and the Atmospheric Entry of Iron Micrometeoroids,” Meteoritics & Planetary Science 51, no. 6 (June 2016): 1063–81, doi:10.1111/maps.12645.
- Andrew G. Tomkins et al., “Ancient Micrometeorites Suggestive of an Oxygen-Rich Archean Upper Atmosphere,” Nature 533 (May 11, 2016): 235–38, doi:10.1038/nature17678.
- James Farquhar, Huiming Bao, and Mark Thiemens, “Atmospheric Influence of Earth’s Earliest Sulfur Cycle,” Science 289, no. 5480 (August 4, 2000): 756–58, doi:10.1126/science.289.5480.756; A. A. Pavlov and J. F. Kasting, “Mass-Independent Fractionation of Sulfur Isotopes in Archean Sediments: Strong Evidence for an Anoxic Archean Atmosphere,” Astrobiology 2, no. 1 (March 2002): 27–41, doi:10.1089/153110702753621321; K. Zahnle, M. Claire, and D. Catling, “The Loss of Mass-Independent Fractionation in Sulfur Due to a Palaeoproterozoic Collapse of Atmospheric Methane,” Geobiology 4, no. 4 (October 2006): 271–83, doi:10.1111/j.1472-4669.2006.00085.x.
- Yoshiki Kanzaki and Takashi Murakami, “Estimates of Atmospheric CO2 in the Neoarchean-Paleoproterozoic from Paleosols,” Geochimica et Cosmochimica Acta 159 (June 15, 2015): 190–219, doi:10.1016/j.gca.2015.03.011.
- Joshua Krissansen-Totton, Giada N. Arney, and David C. Catling, “Constraining the Climate and Ocean pH of the Early Earth with a Geological Carbon Cycle Model,” Proceedings of the National Academy of Sciences USA 115, no. 16 (April 17, 2018): 4105–110, doi:10.1073/pnas.1721296115.
- Lehmer et al, “Atmospheric CO2 Levels.”
- Sanjoy M. Som et al., “Earth’s Air Pressure 2.7 Billion Years Ago Constrained to Less Than Half of Modern Levels,” Nature Geoscience 9 (May 9, 2016): 448–51, doi:10.1038/ngeo2713.
- Sanjoy M. Som et al., “Air Density 2.7 Billion Years Ago Limited to Less Than Twice Modern Levels by Fossil Raindrop Imprints,” Nature 484 (March 28, 2012): 359–62, doi:10.1038/nature10890.
- Lehmer et al, “Atmospheric CO2 Levels.”
- Ross, Improbable Planet.
- Krissansen-Totton, Arney, and Catling, “Constraining the Climate and Ocean pH.”





