Oxygen and Carbon Dioxide History Affirms Divine Creation
Scientific advance continues to reveal details of God’s involvement in the history of Earth’s early life. Research shows that at the just-right times, Earth has received the just-right amounts of atmospheric oxygen necessary for new life-forms.
Earth’s atmosphere has undergone long periods of oxygenation. This oxygenation history dramatically impacts Earth’s life. Some microbial species can flourish without any atmospheric oxygen at all. Many other microbial species can exist and thrive at atmospheric oxygen levels below 1%. A few animal species with individual body sizes below 1 millimeter can survive at atmospheric oxygen levels of only a few percent. However, animals with individual body sizes greater than a few centimeters require atmospheric oxygen levels of at least 8%.
Previous Attempts to Determine Atmospheric Oxygen History
Geochemists have determined the past history of Earth’s atmospheric oxygen level through measurements of isotope ratios and the abundances of certain isotopes. These isotope ratios and isotope abundances serve as proxies (substitutes) for the level of oxygen in the atmosphere. Figure 1 shows Earth’s atmospheric oxygen level throughout Earth’s history determined from these proxies.
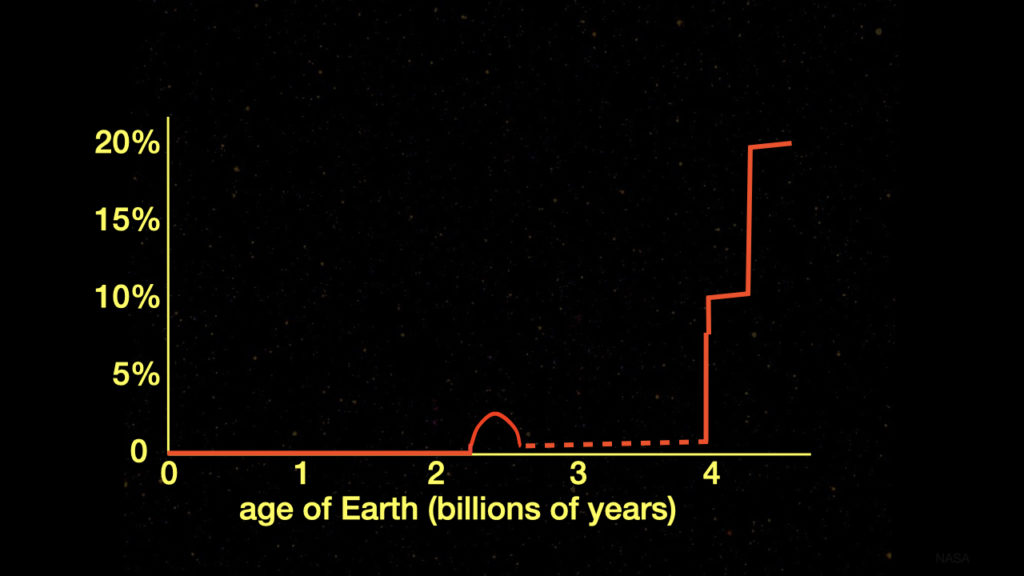
Figure 1: Previous Best Measure of Atmospheric Oxygen Levels throughout Earth’s History
Diagram credit: Hugh Ross
Cerium as a Proxy for Oxygen
The dotted line in figure 1 indicates that part of Earth’s history where geochemists lacked good, undisputed proxies. The best proxies they had were measurements of cerium abundance levels and chromium isotopes. Cerium is preferentially scavenged from seawater under high atmospheric oxygen levels.1 However, cerium oxidation kinetics are poorly constrained and the oxidative pathway has been hotly debated.2 Consequently, based on cerium abundances, atmospheric oxygen level estimates from 2.5–4.0 billion years after Earth’s formation range from 0.02–0.2%.3
Chromium as a Proxy for Oxygen
Oxidative weathering of continental landmasses delivers fractionated chromium to seawater. Therefore, the chromium isotope ratios in marine sediments yield a measure of the oxygen level in the atmosphere. Based on chromium isotopes in two studies, atmospheric oxygen levels from 2.5–4.0 billion years after Earth’s formation yielded values of 0.02–0.2%.4 Two other studies produced values of 0.2–2.0%.5
These disparate values explain why scientists have engaged in such wide-ranging speculations about life during this period. Some have speculated that there may have been brief episodes in the 2.5–4.0-billion-year range of Earth’s age where the atmospheric oxygen level may have been much higher than 2%. In such cases, perhaps plants and animals with body sizes larger than a centimeter may have appeared. Such speculations have caused some evolutionary biologists to conclude that the Avalon (about 575 million years ago) and Cambrian (about 541 million years ago) explosions of life may not have been so explosive. If that is the case, then perhaps God is not as involved in the history of life on Earth as many theists think and claim.
New Measurement of Earth’s Oxygenation History
An international team of a dozen geophysicists and geochemists led by Changle Wang developed a new proxy for determining the atmospheric oxygen level.6 That proxy is the iron isotope ratios in iron-rich sedimentary rocks deposited on continental shelves. The team discovered that regardless of the source of iron-II, partial iron-II oxidation in such rocks requires low oxygen levels in shallow marine waters. In turn, low oxygen levels in shallow marine waters are linked to low atmospheric oxygen levels.
Iron isotope ratios proved to be a powerful, reliable proxy for atmospheric oxygen levels mainly because iron is ubiquitous and abundant in marine sedimentary rocks. Therefore, for the first time scientists possess an accurate and complete record of Earth’s atmospheric oxygen levels from 2.5–4.0 billion years after Earth’s birth (2.1–0.6 billion years ago). No longer is it a dotted line in the graph (see figure 2).
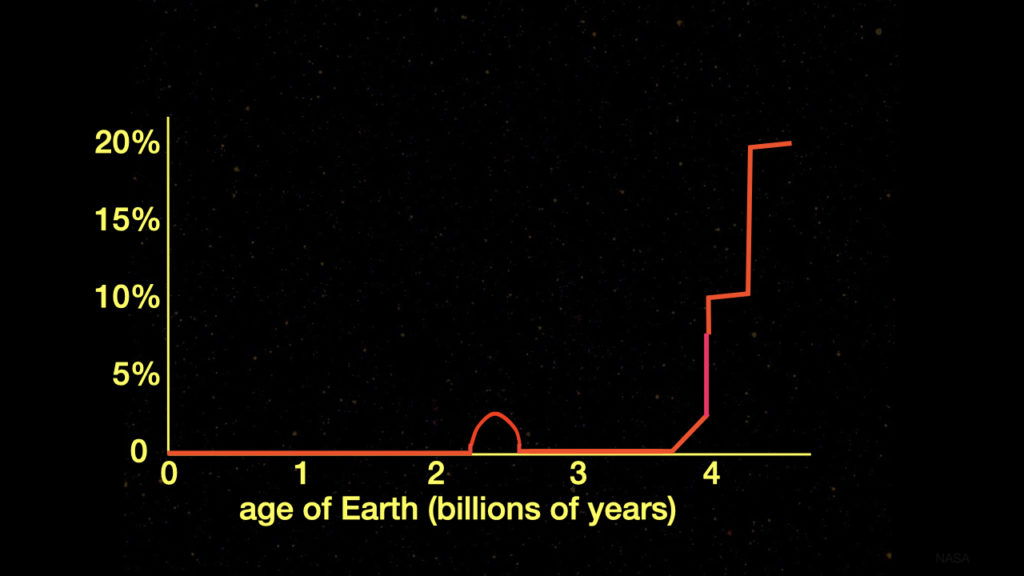
Figure 2: New Measure of Atmospheric Oxygen Levels throughout Earth’s History
Diagram credit: Hugh Ross
Wang’s team established that from 2.1–0.9 billion years ago, Earth’s atmospheric oxygen level remained below 0.2%. Somewhere between 900 and 750 million years ago the atmospheric oxygen level slowly began to rise above 0.2%.
The most recent measurement made by Wang’s team revealed levels from 750 million years ago. The atmospheric oxygen level at that time was about 1%. This level is consistent with the first appearance of eukaryotic predation—tiny primitive animals that feed on other even tinier animals that require a minimum atmospheric oxygen level of 1%. Not until the Avalon explosion at 575 million years ago do scientists see animals in the fossil record that require significantly more atmospheric oxygen.
Earth’s Carbon Dioxide History
Animals also require low atmospheric carbon dioxide levels. High atmospheric carbon dioxide concentrations produce acidosis (low pH) in oceans, lakes, and rivers and in aquatic animal tissues and body fluids. Acidosis is damaging to the long-term health of all aquatic animals and the short-term health of aquatic animals with high metabolic rates.7 For air-breathing animals, elevated carbon dioxide levels disrupt respiration and autoregulation of blood supply, which brings on cognitive and respiratory failure and circulatory arrest.8
It took three slushball events (ice ages where more than half of Earth’s surface is covered with thick ice) to bring the atmospheric carbon dioxide level down to where large-bodied animals could survive.9 These three events were the Sturtian (715–680 million years ago), Marinoan (650–635 million years ago), and Gaskiers (579.9–579.6 million years ago) glaciations. Shortly after the Gaskiers glaciation, Earth’s atmospheric carbon dioxide level finally decreased to a level where large-bodied animals could possibly exist. The first Avalon explosion 575 million years ago marks the first appearance of large-bodied animals.
Philosophical/Theological Implications
The research achievements by Wang’s team close the door on speculations about Earth’s past atmospheric oxygen levels. The last words in their paper are “Surface O2 levels were changing in step with eukaryotic evolution in the Proterozoic.”10 In other words, the moment that the atmospheric oxygen level rose to a level permitting the existence of animals requiring at least that level of atmospheric oxygen, at that same moment those animals appeared. There was no drawn-out evolutionary process. The animals appeared as soon as oxygen conditions permitted their existence.
All naturalistic processes (natural selection, mutations, gene exchange, epigenetics) for driving the evolution of life require long time periods to produce any significant changes. However, the fossil record—in combination with the history of Earth’s atmospheric oxygen levels established by Wang’s team—demonstrates that the time periods are extremely brief, indistinguishable from instantaneous. The immediacy with which oxygen-demanding animals appear when atmospheric oxygen levels attain the minimum levels necessary for their survival supports the argument that a supernatural, superintelligent Creator authored such life, as described in Psalm 104.
Check out more from Reasons to Believe @Reasons.org
Endnotes
- Michael Bau and Andrea Koschinsky, “Oxidative Scavenging of Cerium on Hydrous Fe Oxide: Evidence from the Distribution of Rare Earth Elements and Yttrium between Fe Oxides and Mn Oxides in Hydrogenetic Ferromanganese Crusts,” Geochemical Journal 43, no. 1 (2009): 37–47, doi:10.2343/geochemj.1.0005.
- Bau and Koschinsky, “Oxidative Scavenging of Cerium”; James W. Moflett, “The Relationship between Cerium and Manganese Oxidation in the Marine Environment,” Limnology and Oceanography 39, no. 6 (September 1994): 1309–18, doi:10.4319/lo.1994.39.6.1309.
- Eric J. Bellefroid et al., “Constraints on Paleoproterozoic Atmospheric Oxygen Levels,” Proceedings of the National Academy of Sciences USA 115, no. 32 (July 23, 2018): 8104–9, doi:10.1073/pnas.1806216115; Xiao-Ming Liu et al., “A Persistently Low Level of Atmospheric Oxygen in Earth’s Middle Age,” Nature Communications 12 (January 13, 2021): id. 351, doi:10.1038/s41467-020-20484-7.
- Noah J. Planavsky et al., “Low Mid-Proterozoic Atmospheric Oxygen Levels and the Delayed Rise of Animals,” Science 346, no. 6209 (October 31, 2014): 635–8, doi:10.1126/science.1258410; Devon B. Cole et al., “A Shale-Hosted Cr Isotope Record of Low Atmospheric Oxygen during the Proterozoic,” Geology 44, no. 7 (July 1, 2016): 555–8, doi:10.1130/G37787.1.
- Donald E. Canfield et al., “Highly Fractionated Chromium Isotopes in Mesoproterozoic-Aged Shales and Atmospheric Oxygen,” Nature Communications 9 (July 20, 2018): id. 2871, doi:10.1038/s41467-018-05263-9; G. J. Gilleaudeau et al., “Oxygenation of the Mid-Proterozoic Atmosphere: Clues from Chromium Isotopes in Carbonates,” Geochemistry Perspectives Letters 2, no. 2 (May 24, 2016): 178–87, doi:10.7185/geochemlet.1618.
- Changle Wang et al., “Strong Evidence for a Weakly Oxygenated Ocean-Atmosphere System during the Proterozoic,” Proceedings of the National Academy of Sciences USA 119, no. 6 (January 31, 2022): id. e2116101119, doi:10.1073/pnas.2116101119.
- Hans O. Pörtner, Martina Langenbuch, and Anke Reipschläger, “Biological Impact of Elevated Ocean CO2 Concentrations: Lessons from Animal Physiology and Earth History,” Journal of Oceanography 60, no. 4 (August 1, 2004): 705–18, doi:10.1007/s10872-004-5763-0.
- Zaher S. Azzam et al., “The Physiological and Molecular Effects of Elevated CO2 Levels,” Cell Cycle 9, no. 8 (April 20, 2010): 1528–32, doi:10.4161/cc.9.8.11196; Kris Permentier et al., “Carbon Dioxide Poisoning: A Literature Review of an Often Forgotten Cause of Intoxication in the Emergency Department,” International Journal of Emergency Medicine 10 (April 4, 2017): id. 14, p. 1, doi:10.1186/s12245-017-0142-y.
- Stephan V. Sobolev and Michael Brown, “Surface Erosion Events Controlled the Evolution of Plate Tectonics on Earth,” Nature 570 (June 5, 2019): 52–57, doi:10.1038/s41586-019-1258-4; Hugh Ross, “Why Do We Need Snowball Events?” Today’s New Reason to Believe (blog), Reasons to Believe, August 5, 2019.
- Wang et al., “Strong Evidence,” p. 6.





