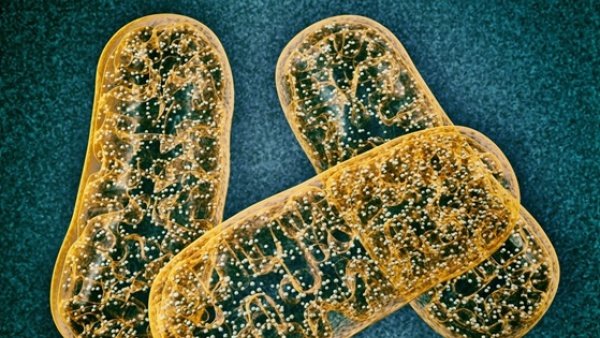
Evolutionary Paradigm Lacks Explanation for Origin of Mitochondria and Eukaryotic Cell
You carried the cross
Of my shame
Oh my shame
You know I believe it
But I still haven’t found
What I’m looking for—Adam Clayton, Dave Evans, Larry Mullen, Paul David Hewson, Victor Reina
One of my favorite U2 songs is “I Still Haven’t Found What I’m Looking For.” For me, it’s a reminder that because of Christ, my life has meaning, purpose, and a sense of destiny. Still, I will never discover ultimate fulfillment in this world no matter how hard I search, but in the world to come—the new heaven and new earth.
Though their pursuit is scientific and not religious, many scientists have also failed to find what they have been looking for. Physicists are on a quest to find the Theory of Everything—a Grand Unified Theory (GUT) that can account for everything in physics. However, a GUT eludes them.
On the other hand, life scientists appear to have found it. They claim to have discovered biology’s GUT: the theory of evolution. Many biologists assert that evolutionary mechanisms can fully account for the origin, history, and design of life. And they are happy to sing about their discovery any chance they get.
Yet, despite this claim, the evolutionary paradigm seems to come up short time and time again when it comes to explaining key events in life’s history. And this failure serves as the basis for my skepticism regarding the evolutionary paradigm.
Currently, evolutionary biologists lack explanations for the key transitions in life’s history, including thes
- origin of life,
- origin of eukaryotic cells,
- origin of sexual reproduction,
- origin of body plans,
- origin of consciousness,
- and the origin of human exceptionalism.
To be certain, evolutionary biologists have proposed models to explain each of these transitions, but the models consistently fail to deliver, as a recent review article published by two prominent evolutionary biologists from the Hungarian Academy of Sciences illustrates.1 In this article, these researchers point out the insufficiency of the endosymbiont hypothesis—the leading evolutionary model for the origin of eukaryotic cells—to account for the origin of mitochondria and, hence, eukaryogenesis.
Lynn Margulis (1938–2011) advanced the endosymbiont hypothesis for the origin of eukaryotic cells in the 1960s, building on the ideas of Russian botanist, Konstantin Mereschkowski. Taught in introductory high school and college biology courses, Margulis’s work has become a cornerstone idea of the evolutionary paradigm. This classroom exposure explains why students often ask me about the endosymbiont hypothesis when I speak on university campuses. Many first-year biology students and professional life scientists alike find the evidence for this idea compelling and, consequently, view it as providing broad support for an evolutionary explanation for the history and design of life.
According to the hypothesis, complex cells originated when symbiotic relationships formed among single-celled microbes after free-living bacterial and/or archaeal cells were engulfed by a “host” microbe. (Ingested cells that take up permanent residence within other cells are referred to as endosymbionts.)
Presumably, organelles such as mitochondria were once endosymbionts. Once engulfed, the endosymbionts took up permanent residency within the host, with the endosymbiont growing and dividing inside the host. Over time, the endosymbionts and the host became mutually interdependent, with the endosymbionts providing a metabolic benefit for the host cell. The endosymbionts gradually evolved into organelles through a process referred to as genome reduction. This reduction resulted when genes from the endosymbionts’ genomes were transferred into the genome of the host organism. Eventually, the host cell evolved the machinery to produce the proteins needed by the former endosymbiont and processes to transport those proteins into the organelle’s interior.
Evidence for the Endosymbiont Hypothesis
The similarity between organelles and bacteria serve as the main line of evidence for the endosymbiont hypothesis. For example, mitochondria—which are believed to be descended from a group of alpha-proteobacteria—are about the same size and shape as a typical bacterium and have a double membrane structure like gram-negative cells. These organelles also divide in a way that is reminiscent of bacterial cells.
Biochemical evidence also exists for the endosymbiont hypothesis. Evolutionary biologists view the presence of the diminutive mitochondrial genome as a vestige of this organelle’s evolutionary history. They see the biochemical similarities between mitochondrial and bacterial genomes as further evidence for the evolutionary origin of these organelles.
The presence of the unique lipid, cardiolipin, in the mitochondrial inner membrane also serves as evidence for the endosymbiont hypothesis. This important lipid component of bacterial inner membranes is not found in the membranes of eukaryotic cells—except for the inner membranes of mitochondria. In fact, biochemists consider it a signature lipid for mitochondria and a vestige of this organelle’s evolutionary history.2
Does the Endosymbiont Hypothesis Successfully Account for the Origin of Mitochondria?
Despite the seemingly compelling evidence for the endosymbiont hypothesis, evolutionary biologists lack a genuine explanation for the origin of mitochondria, and, in a broader context, the origin of eukaryotic cells. In their recently published critical review, Zachar and Szathmary point out that evolutionary biologists have proposed over twenty different evolutionary scenarios for the mitochondrial origins that umbrella underneath the endosymbiont hypothesis. Of these, they identify eight that are reasonable, casting the others aside. Still, these eight hypotheses fail to fully account for the origin of mitochondria. The Hungarian biologists delineate twelve questions that any successful endosymbiogenesis model must answer. In turn, they demonstrate that none of these models answers all the questions. In doing so, the two researchers call for a new theory.
In the article’s abstract, the authors state, “The origin of mitochondria is a unique and hard evolutionary problem, embedded within the origin of eukaryotes. . . . Contending theories widely disagree on ancestral partners, initial conditions and unfolding events. There are many open questions but there is no comparative examination of hypotheses. We have specified twelve questions about the observable facts and hidden processes leading to the establishment of the endosymbiont that a valid hypothesis must address. There is no single theory capable of answering all questions.”3
Space doesn’t permit me to discuss each of the questions posed by the pair of biologists. Still, I would like to call attention to a few problems confronting the endosymbiont hypothesis, highlighted in their critical review.
Lack of Transitional Intermediates. Biologists have yet to discover any single-celled organisms that represent transitional intermediates between prokaryotes and eukaryotic cells. (There are some eukaryotes that lack mitochondria, but they appear to have lost these organelles.) All complex cells display the eukaryotic hallmark features. In other words, it looks as if eukaryotic cells emerged in a short period of time, without any transitional forms. In fact, some biologists dub the transition the eukaryotic big bang.
Chimeric Nature of Eukaryotic Cells. Eukaryotic cells possess an unusual combination of features. Their information-processing systems resemble those of archaea, but their membranes and energy metabolism are bacteria-like. There is no plausible evolutionary scenario to explain this blend of features. It would require the archaeon host to replace its membranes while retaining all its information-processing genes. Evolutionary biologists know of no instance in which this type of transition took place, nor do they know how it could have occurred.
Absence of Membrane Bioenergetics in the Host. All prokaryotic organisms rely on their plasma membrane to produce energy. If eukaryotic cells emerged via endosymbiogenesis, then the plasma membranes of eukaryotic cells should possess vestiges of that past function. Yet, the plasma membranes of eukaryotic cells show no traces of this essential biochemical feature.
Mechanism of Inclusion. The most plausible way for the endosymbiont to be taken up by the host cell is through a process called phagocytosis. But why wouldn’t the engulfed cell be digested by the host? How did the endosymbiont escape destruction? And, if it somehow survived, why doesn’t the mitochondria possess a triple membrane system, with the outermost membrane derived from the phagosome?
Early Selective Advantage. Once inside the host, why didn’t the endosymbiont simply reproduce, overrunning the host cell? What benefit would it be for the host cell to initially harbor the endosymbiont? Currently, evolutionary biologists don’t have answers to troubling questions such as these.
The challenges delineated by the Hungarian biologists aren’t the only ones faced by evolutionary models for endosymbiogenesis. As I discuss in a previous article, mitochondrial protein biogenesis poses another difficult problem for the endosymbiont hypothesis.
The authors of the critical review sum it up this way: “The integration of mitochondria was a major transition, and a hard one. It poses puzzles so complicated that new theories are still generated 100 years since endosymbiogenesis was first proposed by Konstantin Mereschkowsky and 50 years since Lynn Margulis cemented the endosymbiotic origin of mitochondria into evolutionary biology. . . . One would expect that by this time, there is a consensus about the transition, but far from that even the most fundamental points are still debated.”4
Though evolutionary biologists claim to have life’s history all figured out, in reality they are like most of us—they still haven’t found what they are looking for.
Resources
- “Why Do Mitochondria Have DNA?” by Fazale Rana (article)
- “Mitochondrial Genomes: Evidence for Evolution or Creation?” by Fazale Rana (article)
- “Can a Creation Model Explain the Origin of Mitochondria?” by Fazale Rana (article)
- “Complex Protein Biogenesis Hints at Intelligent Design” by Fazale Rana (article)
- “Biology’s Big Bangs” by Fazale Rana (article)
Endnotes
- Istvan Zachar and Eors Szathmary, “Breath-Giving Cooperation: Critical Review of Origin of Mitochondria Hypotheses,” Biology Direct 12 (August 14, 2017): 19, doi:10.1186/s13062-017-0190-5.
- In previous posts (here, here, and here), I explain the rationale for mitochondrial DNA and the presence of cardiolipin in the inner mitochondrial membrane from a creation model/intelligent design vantage point and, in doing so, demonstrate that the two biochemical features aren’t uniquely explained by the endosymbiont hypothesis.
- Zachar and Szathmary, “Breath-Giving Cooperation.”
- Zachar and Szathmary, “Breath-Giving Cooperation.”
Subjects: Cells, Challenges to Evolution, Origin of Life