Protein Amino Acids Form a “Just-Right” Set of Biological Building Blocks
Like most kids, I had a set of Lego building blocks. But, growing up in the 1960s, the Lego sets were nothing like the ones today. I am amazed at how elaborate and sophisticated Legos have become, consisting of interlocking blocks of various shapes and sizes, gears, specialty parts, and figurines—a far cry from the square and rectangular blocks that made up the Lego sets of my youth. The most imaginative things I could ever hope to build were long walls and high towers.
It goes to show: the set of building blocks make all the difference in the world.
This truism applies to the amino acid building blocks that make up proteins. As it turns out, proteins are built from a specialty set of amino acids that have the just-right set of properties to make life possible, as recent work by researchers from Germany attests.1 From my vantage point as a biochemist and a Christian, the just-right amino acid composition of proteins evinces intelligent design and is part of the reason I think a Creator must have played a direct role in the origin and design of life.
Why is the Same Set of Twenty Amino Acids Used to Build Proteins?
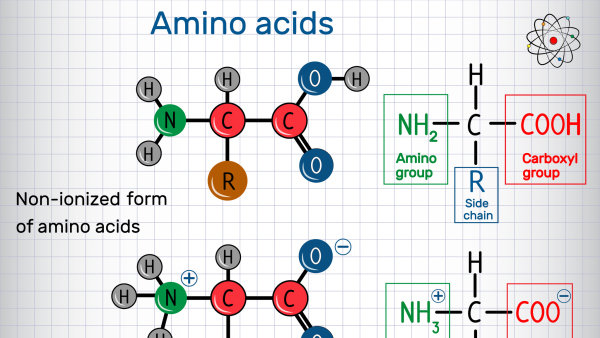
It stands as one of the most important insights about protein structure discovered by biochemists: The set of amino acids used to build proteins is universal. In other words, the proteins found in every organism on Earth are made up of the same 20 amino acids.
Yet, hundreds of amino acids exist in nature. And, this abundance prompts the question: Why these 20 amino acids? From an evolutionary standpoint, the set of amino acids used to build proteins should reflect:
1) the amino acids available on early Earth, generated by prebiotic chemical reactions;
2) the historically contingent outworking of evolutionary processes.
In other words, evolutionary mechanisms would have cobbled together an amino acid set that works “just good enough” for life to survive, but nothing more. No one would expect evolutionary processes to piece together a “just-right,” optimal set of amino acids. In other words, if evolutionary processes shaped the amino acid set used to build proteins, these biochemical building blocks should be much like the unsophisticated Lego sets little kids played with in the 1960s.
An Optimal Set of Amino Acids
But, contrary to this expectation, in the early 1980s biochemists discovered that an exquisite molecular rationale undergirds the amino acid set used to make proteins. Every aspect of the amino acid structure has to be precisely the way it is for life to be possible. On top of that, researchers from the University of Hawaii have conducted a quantitative comparison of the range of chemical and physical properties possessed by the 20 protein-building amino acids versus random sets of amino acids that could have been selected from early Earth’s hypothetical prebiotic soup.2 They concluded that the set of 20 amino acids is optimal. It turns out that the set of amino acids found in biological systems possesses the “just-right” properties that evenly and uniformly vary across a broad range of size, charge, and hydrophobicity. They also showed that the amino acids selected for proteins are a “highly unusual set of 20 amino acids; a maximum of 0.03% random sets outperformed the standard amino acid alphabet in two properties, while no single random set exhibited greater coverage in all three properties simultaneously.”3
A New Perspective on the 20 Protein Amino Acids
Beyond charge, size, and hydrophobicity, the German researchers wondered if quantum mechanical effects play a role in dictating the universal set of 20 protein amino acids. To address this question, they examined the gap between the HOMO (highest occupied molecular orbital) and the LUMO (lowest unoccupied molecular orbital) for the protein amino acids. The HOMO-LUMO gap is one of the quantum mechanical determinants of chemical reactivity. More reactive molecules have smaller HOMO-LUMO gaps than molecules that are relatively nonreactive.
The German biochemists discovered that the HOMO-LUMO gap was small for 7 of the 20 amino acids (histidine, phenylalanine cysteine, methionine, tyrosine, and tryptophan), and hence, these molecules display a high level of chemical activity. Interestingly, some biochemists think that these 7 amino acids are not necessary to build proteins. Previous studies have demonstrated that a wide range of foldable, functional proteins can be built from only 13 amino acids (glycine, alanine, valine, leucine, isoleucine, proline, serine, threonine, aspartic acid, glutamic acid, asparagine, lysine, and arginine). As it turns out, this subset of 13 amino acids has a relatively large HOMO-LUMO gap and, therefore, is relatively unreactive. This suggests that the reactivity of histidine, phenylalanine cysteine, methionine, tyrosine, and tryptophan may be part of the reason for the inclusion of the 7 amino acids in the universal set of 20.
As it turns out, these amino acids readily react with the peroxy free radical, a highly corrosive chemical species that forms when oxygen is present in the atmosphere. The German biochemists believe that when these 7 amino acids reside on the surface of proteins, they play a protective role, keeping the proteins from oxidative damage.
As I discussed in a previous article, these 7 amino acids contribute in specific ways to protein structure and function. And they contribute to the optimal set of chemical and physical properties displayed by the universal set of 20 amino acids. And now, based on the latest work by the German researchers, it seems that the amino acids’ newly recognized protective role against oxidative damage adds to their functional and structural significance in proteins.
Interestingly, because of the universal nature of biochemistry, these 7 amino acids must have been present in the proteins of the last universal common ancestor (LUCA) of all life on Earth. And yet, there was little or no oxygen present on early Earth, rendering the protective effect of these amino acids unnecessary. The importance of the small HOMO-LUMO gaps for these amino acids would not have become realized until much later in life’s history when oxygen levels became elevated in Earth’s atmosphere. In other words, inclusion of these amino acids in the universal set at life’s start seemingly anticipates future events in Earth’s history.
Protein Amino Acids Chosen by a Creator
The optimality, foresight, and molecular rationale undergirding the universal set of protein amino acids is not expected if life had an evolutionary origin. But, it is exactly what I would expect if life stems from a Creator’s handiwork. As I discuss in The Cell’s Design, objects and systems created and produced by human designers are typically well thought out and optimized. Both are indicative of intelligent design. In human designs, optimization is achieved through foresight and planning. Optimization requires inordinate attention to detail and careful craftsmanship. By analogy, the optimized biochemistry, epitomized by the amino acid set that makes up proteins, rationally points to the work of a Creator.
Resources
- The Cell’s Design: How Chemistry Reveals the Creator’s Artistry by Fazale Rana (book)
- “Why These 20 Amino Acids?” by Fazale Rana (article)
Endnotes
- Matthias Granhold et al., “Modern Diversification of the Amino Acid Repertoire Driven by Oxygen,” Proceedings of the National Academy of Sciences USA 115 (January 2, 2018): 41–46, doi:10.1073/pnas.1717100115.
- Gayle K. Philip and Stephen J. Freeland, “Did Evolution Select a Nonrandom ‘Alphabet’ of Amino Acids?” Astrobiology 11 (April 2011): 235–40, doi:10.1089/ast.2010.0567.
- Philip and Freeland, “Did Evolution Select,” 235–40.
Check out more from Dr. Fazale Rana @Reasons.org