Do Plastic-Eating Bacteria Dump the Case for Creation?
At the risk of stating the obvious: Plastics are an indispensable part of our modern world. Yet, plastic materials cause untold problems for the environment. One of the properties that makes plastics so useful also makes them harmful. Plastics don’t readily degrade.
Recently, researchers discovered a new strain of bacteria that had recently evolved the ability to degrade plastics. These microbes may help solve some of the environmental problems caused by plastics, but their evolution seemingly causes new problems for people who hold the view that a Creator is responsible for life’s origin and design. But, is this really the case? To find out, we need to break down this discovery.
One plastic in widespread use today is polyethylene terephthalate (PET). This polymer was patented in the 1940s and became widely used in the 1970s. Most people are familiar with PET because it is used to make drinking bottles.
This material is produced by reacting ethylene glycol with terephthalic acid (both produced from petroleum). Crystalline in nature, this plastic is a durable material that is difficult to break down, because of the inaccessibility of the ester linkages that form between the terephthalic acid and ethylene glycol subunits that make up the polymer backbone.
PET can be recycled, thereby mitigating its harmful effects on the environment. A significant portion of PET is mechanically recycled by converting it into fibers used to manufacture carpets.
In principle, PET could be recycled by chemically breaking the ester linkages holding the polymer together. When the ester linkages are cleaved, ethylene glycol and terephthalic acid are the breakdown products. These recovered starting materials could be reused to make more PET. Unfortunately, chemical recycling of PET is expensive and difficult to carry out because of the inaccessibility of the ester linkages. In fact, it is cheaper to produce PET from petroleum products than from the recycled monomers.
Can Bacteria Recycle PET?
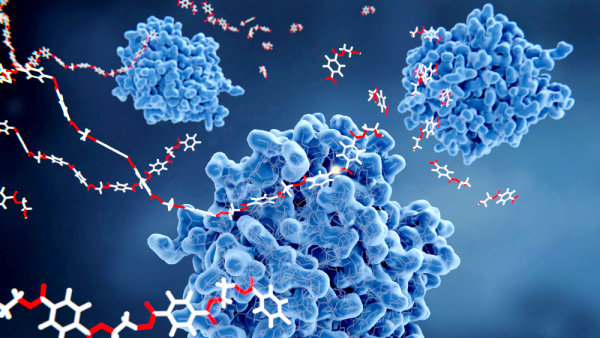
An interesting advance took place in 2016 that has important implications for PET recycling. A team of Japanese researchers discovered a strain of the bacterium Ideonella sakaiensis that could break down PET into terephthalic acid and ethylene glycol.1 This strain was discovered by screening wastewater, soil, sediments, and sludge from a PET recycling facility. The microbe produces two enzymes, dubbed PETase and MHETase, that work in tandem to convert PET into its constituent monomers.
Evolution in Action
Researchers think that this microbe acquired DNA from the environment or another microbe via horizontal gene transfer. Presumably, this DNA fragment harbored the genes for cutinase, an enzyme that breaks down ester linkages. Once the I. sakaiensis strain picked up the DNA and incorporated it into its genome, the cutinase gene must have evolved so that it now encodes the information to produce two enzymes with the capacity to break down PET. Plus, this new capability must have evolved rather quickly, over the span of a few decades.
PETase Structure and Evolution
In an attempt to understand how PETase and MHETase evolved and how these two enzymes might be engineered for recycling and bioremediation purposes, a team of investigators from the University of Plymouth determined the structure of PETase with atomic level detail.2 They learned that this enzyme has the structural components characteristic of a family of enzymes called alpha/beta hydrolases. Based on the amino acid sequence of the PETase, the researchers concluded that its closest match to any existing enzyme is to a cutinase produced by the bacterium Thermobifida fusca. One of the most significant differences between these two enzymes is found at their active sites. (The active site is the location on the enzyme surface that binds the compounds that the enzyme chemically alters.) The active site of the PETase is broader than the T. fusca cutinase, allowing it to accommodate PET polymers.
As researchers sought to understand how PETase evolved from cutinase, they engineered amino acid changes in PETase, hoping to revert it to a cutinase. To their surprise, the resulting enzyme was even more effective at degrading PET than the PETase found in nature.
This insight does not help explain the evolutionary origin of PETase, but the serendipitous discovery does point the way to using engineered PETases for recycling and bioremediation. One could envision spraying this enzyme (or the bacterium I. sakaiensis) onto a landfill or in patches of plastics floating in the Earth’s oceans. Or alternatively using this enzyme at recycling facilities to generate the PET monomers.
As a Christian, I find this discovery exciting. Advances such as these will help us do a better job as planetary caretakers and as stewards of God’s creation, in accord with the mandate given to us in Genesis 1.
But, this discovery does raise a question: Does the evolution of a PET-eating bacterium prove that evolution is true? Does this discovery undermine the case for creation? After all, it is evolution happening right before our eyes.
Is Evolution in Action Evidence for Evolution?
To answer this question, we need to recognize that the term “evolution” can take on a variety of meanings. Each one reflects a different type of biological transformation (or presumed transformation).
It is true that organisms can change as their environment changes. This occurs through mutations to the genetic material. In rare circumstances, these mutations can create new biochemical and biological traits, such as the ones that produced the strain of I. sakaiensis that can degrade PET. If these new traits help the organism survive, it will reproduce more effectively than organisms lacking the trait. Over time, this new trait will take hold in the population, causing a transformation of the species.
And this is precisely what happened with I. sakaiensis. However, microbial evolution is not controversial. Most creationists and intelligent design proponents acknowledge evolution at this scale. In a sense, it is not surprising that single-celled microbes can evolve, given their extremely large population sizes and capacity to take up large pieces of DNA from their surroundings and incorporate it into their genomes.
Yet, I. sakaiensis is still I. sakaiensis. In fact, the similarity between PETase and cutinases indicates that only a few amino acid changes can explain the evolutionary origin of new enzymes. Along these lines, it is important to note that both cutinase and PETase cleave ester linkages. The difference between these two enzymes involves subtle structural differences triggered by altering a few amino acids. In other words, the evolution of a PET-degrading bacterium is easy to accomplish through a form of biochemical microevolution.
But just because microbes can undergo limited evolution at a biochemical level does not mean that evolutionary mechanisms can account for the origin of biochemical systems and the origin of life. That is an unwarranted leap. This study is evidence for microbial evolution, nothing more.
Though this advance can help us in our planetary stewardship role, this study does not provide the type of evidence needed to explain the origin of biochemistry and, hence, the origin of life through evolutionary means. Nor does it provide the type of evidence needed to explain the evolutionary origin of life’s major groups. Evolutionary biologists must develop appropriate evidence for these putative transformations, and so far, they haven’t.
Evidence of microbial evolution in action is not evidence for the evolutionary paradigm.
Resources:
- Creating Life in the Lab: How New Discoveries in Synthetic Biology Make a Case for a Creator by Fazale Rana (book)
- Evolution under the Microscope by Fazale Rana (video)
- “Does the Evolution of Caffeine-Eating Bacteria Stimulate the Case for Biological Evolution?” by Fazale Rana (article)
- “Long-Term Evolution Experiment: Evidence for the Evolutionary Paradigm?” by Fazale Rana (article)
- “Evidence that Humans are Evolving is Not Evidence for Human Evolution” by Fazale Rana (article)
- “Evolution: Seeing Isn’t Believing” by Fazale Rana (article)
Check out more from Dr. Fazal Rana @reasons.org