Molecular Logic of the Electron Transport Chain Supports Creation
“It was said that some scientists attended the oxidative phosphorylation sessions of the Federation meetings because they knew a good punch up was on the cards.”
—John Prebble, Department of Biological Sciences, University of London
It has been described as one of the most “heated and acrimonious debates in biochemistry during the twentieth century,”1 and its resolution carries implications for a different ideological conflict—that of the origin of life.
This battle royale (dubbed the Ox Phos Wars) took place in the 1960s and early 1970s. At that time, biochemists were trying to decipher the mechanism used by mitochondria to produce the high-energy compound called ATP (adenosine triphosphate) through a process called oxidative phosphorylation (Ox Phos for short). Many components of the cell’s machinery use ATP to power their operations.
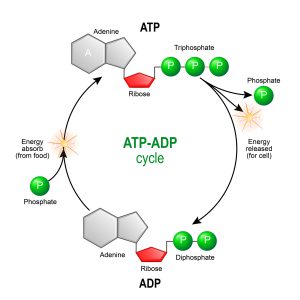
Figure 1: A schematic of the synthesis and breakdown cycle of ATP and ADP. Image credit: Shutterstock
So acrimonious was the debate that scientists involved in this controversy often came close to blows when publicly debating the mechanism of oxidative phosphorylation. Much of the controversy centered around an idea known as the chemiosmotic theory, proposed by biochemist Peter Mitchell. He argued that the electron transport chain generates a proton gradient across the mitochondrial inner membrane and, in turn, exploits that gradient through a coupling process to drive the synthesis of ATP from ADP (adenosine diphosphate) and inorganic phosphate. (see figures 1 and 2). (The reverse reaction liberates chemical energy that drives many biochemical processes.)
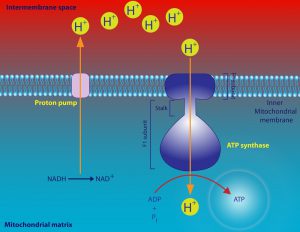
Figure 2: A schematic of the chemiosmotic theory. Image credit: Shutterstock
At the time, this idea was met with a large measure of skepticism by biochemists. It didn’t fit with the orthodoxy, characteristic of classical biochemistry. Biochemists found Mitchell’s ideas hard to understand and his personality abrasive, both of which led to the acrimony.
Origin-of-life researcher Leslie Orgel referred to the chemiosmotic theory as one of the most counterintuitive ideas to ever come out of biology, comparing it to the ideas that formed the foundations of quantum mechanics and relativity.2
Many biochemists preferred the chemical theory of oxidative phosphorylation over Mitchell’s chemiosmotic theory. Researchers thought that the phosphate group added to ADP was transferred from one of the components of the electron transport chain. In an attempt to support this idea, many biochemists frantically searched for a chemical intermediate with a high-energy phosphate moiety that could power the synthesis of ATP.
The chemical theory was based on a process called substrate-level phosphorylation, exemplified by two reactions that form ATP during glycolysis. In one reaction, 1,3-diphosphoglycerate transfers one of its phosphate groups to ADP to form ATP. (In this case, 3-diphosphoglycerate serves as the intermediate with a high-energy phosphate moiety.) In the second reaction, phosphoenolpyruvate transfers a phosphate group to ADP to make ATP, with phosphoenolpyruvate functioning as the intermediate bearing a high-energy phosphate residue. (See figure 3.)
As it turns out, the elusive intermediate was never found, forcing adherents of the chemical theory to abandon their model. Peter Mitchell’s idea won the day. In fact, Mitchell was awarded the Nobel Prize in Chemistry in 1978 for his contribution to understanding the mechanism of oxidative phosphorylation.
Today, biochemists readily recognize the importance of proton gradients and the chemiosmotic process. Proton gradients are pervasive in living systems. Mitochondria are not alone. Chloroplasts rely on proton gradients during the process of photosynthesis. Bacteria and archaea also use proton gradients across their plasma membranes to harvest energy. Cells use proton gradients to transport material across cell membranes. And proton gradients even power the bacterial flagellum.
Now that oxidative phosphorylation is understood, some evolutionary biologists and origin-of-life researchers have turned their attention to two questions: (1) How did chemiosmosis originate? and (2) Why are proton gradients so central to biochemical operations?
Oxidative Phosphorylation and the Evolutionary Paradigm
For many evolutionary biologists, understanding the origin of oxidative phosphorylation (and the use of proton gradients, in general) assumes a position of unique prominence because of the central role this process plays in harvesting energy in both prokaryotic and eukaryotic organisms. In other words, understanding the origin of oxidative phosphorylation (and use of proton gradients) is central to understanding the origin of life and the fundamental design of biochemical systems.
Because the use of proton gradients in living systems is odd and counterintuitive, it becomes tempting for many origin-of-life researchers and evolutionary biologists to conclude that chemiosmosis reflects the outworking of a historically contingent evolutionary process that relied on existing systems and designs that were co-opted and, in turn, modified. This notion becomes reinforced by the work of origin-of-life researcher Nick Lane.
Lane and his collaborators conclude that proton gradients must have been integral to the biochemistry of LUCA (the last universal common ancestor) because proton gradients are a near-universal feature of living systems. If so, then the use of proton gradients must have emerged during the origin-of-life process before LUCA originated. Lane and his team go so far as to propose that the first proto-cells emerged near hydrothermal vents and made use of naturally occurring proton gradients found in these environments as their energy source.3
Once this system was in place, the strategy was retained in the cell lines that diverged from these early proto-cellular entities as the electron transport chain evolved from a simple, naturally occurring vent process to the complex process found in both prokaryotic and eukaryotic organisms. In other words, it would seem that the odd, counterintuitive nature of proton gradients reflects the happenstance outworking of chemical evolution that began when the naturally occurring proton gradients were co-opted in the early stages of chemical evolution.
But Lane’s recent insight indicates that, though counterintuitive, the use of proton gradients to harvest the energy required to make ATP makes sense, displaying an exquisite molecular rationale.4 And if so, it forces a rethink of the explanation for the origin of chemiosmosis. To appreciate this shift in perspective, it is helpful to understand the process of oxidative phosphorylation, beginning with glycolysis and the Kreb’s cycle.
Glycolysis and the Kreb’s Cycle
The glycolytic pathway converts the fuel molecule glucose (a 6-carbon sugar) into two pyruvate molecules (3-carbon). This process proceeds through eleven chemical intermediates and nets 2 molecules of ATP (generated through substrate-level phosphorylation) and two molecules of NADH (nicotinamide adenine dinucleotide). NADH harbors high-energy electrons generated from the energy liberated from the breakdown and oxidation of glucose. As it turns out, the NADH molecules play a central role in generating most of the ATP produced when a sugar molecule breaks down.
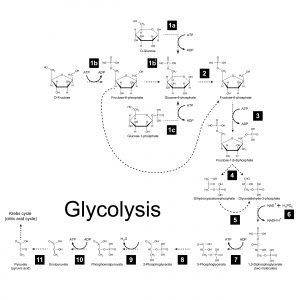
Figure 3: Glycolysis. Image credit: Shutterstock
The pyruvate generated by glycolysis is transported across the mitochondrial inner membrane into the matrix of the organelle. Here pyruvate is transformed into a molecule of carbon dioxide and a 2-carbon intermediate called acetyl CoA. This process generates 2 additional molecules of NADH.
In turn, the Kreb’s cycle converts each acetyl CoA molecule into two molecules of carbon dioxide. (The net reaction: a 6-carbon glucose molecule breaks down into 6 carbon dioxide molecules.) During the process, the breakdown of each acetyl CoA molecule generates 1 ATP molecule (via substrate-level phosphorylation) and 3 molecules of NADH. Additionally, 1 molecule of FADH2 is formed. Like NADH, this molecule possesses high-energy electrons. (See figure 4.)
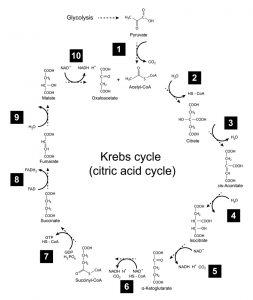
Figure 4: Kreb’s cycle. Image credit: Shutterstock
The Electron Transport Chain and Oxidative Phosphorylation
The high-energy electrons of NADH and FADH2 are transferred to the electron transport chain, which is embedded in the inner membrane.
Four protein complexes (dubbed I, II, III, and IV) make up the electron transport chain. The high-energy electrons from NADH and FADH2 are shuffled from one protein complex to the next, with each transfer releasing energy that is used to transport protons from the mitochondrial matrix across the inner membrane, establishing the proton gradient. (See figure 5.) Oxygen is the final electron acceptor in the electron transport chain. The electrons transferred to oxygen lead to the formation of a water molecule.
Because protons are positively charged, the exterior region outside the inner membrane is positively charged and the interior region is negatively charged. The charge differential created by the proton gradient is analogous to a battery and the inner membrane is like a capacitor.
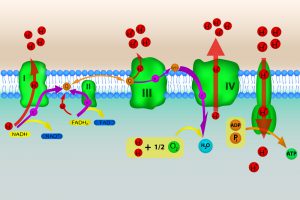
Figure 5: Electron Transport Chain. Image credit: Shutterstock
The coupling of the proton gradient to ATP synthesis occurs as a result of the flow of positively charged protons through the F0 component of a protein complex called F1-F0ATPase(also embedded in the mitochondrial inner membrane). F1-F0ATPase uses this flux to convert electrochemical energy into mechanical energy that, in turn, is used to drive the formation of ATP from ADP and inorganic phosphate.
The Molecular Logic of Proton Gradients
So, why are chemiosmosis and proton gradients universal features of living systems? Are they an outworking of a historically contingent evolutionary process? Or is there something more at work?
Even though proton gradients seem counterintuitive at first glance, the use of proton gradients to power the production of ATP and other cellular processes reflects an underlying ingenuity and exquisite molecular logic. Research shows that proton gradients allow the cell to efficiently extract as much energy as possible from the breakdown of glucose (and other biochemical foodstuffs).5 On the other hand, if ATP was produced exclusively by substrate-level phosphorylation, using a high-energy chemical intermediate, much of the energy liberated from the breakdown of glucose would be lost as heat.
To understand why this is so, consider this analogy. Suppose people in a particular community receive their daily allotment of water in a 10-gallon bucket. The water they receive each day is retrieved from a reservoir with a 12-gallon bucket and then transferred to their bucket. In the process, two gallons of water is lost. Now, suppose the water from the reservoir is retrieved with a 12-gallon bucket but dumped into a secondary reservoir that has a tap. The tap allows each 10-gallon bucket to be filled without losing two gallons. Though the procedure is indirect and more complicated, using the secondary reservoir to distribute water is more efficient in the long run. In the first scenario, it takes 60 gallons of water (transferred from the reservoir in five 12-gallon buckets) to fill up five 10-gallon buckets. In the second scenario, the same amount of water transferred from the reservoir can fill six 10-gallon buckets. With each transfer, the additional two gallons accumulate in the reservoir until there is enough water to fill another 10-gallon bucket.
With substrate-level phosphorylation, when the phosphate group is transferred from the high-energy intermediate to ADP to form ATP, excess energy released during the transfer is lost as heat. It takes 7 kcal/mole of energy to add a phosphate group to ADP to form ATP. Let’s say that the hypothetical chemical intermediate releases 10 kcal/mole when its high-energy phosphate bond is broken. Three kcal/mole of energy is lost.
On the other hand, using the electron transport chain to build up a proton gradient is like the reservoir in our analogy. It allows that extra three kcal/mole to be stored in the proton gradient. We can think of the F1-F0ATPase as analogous to the tap. It uses 7 kcal/mole of energy released when protons flow through its channels to drive the formation of ATP from ADP and inorganic phosphate. The unused energy from the proton gradient continues to accumulate until enough energy is available to form another ATP molecule. So, in our hypothetical scenario, if the cell used substrate-level phosphorylation to make ATP, 70 molecules of the high-energy intermediate would yield 70 molecules of ATP with 210 kcal/mole of energy released as heat. But, using the electron transport chain to generate proton gradients yields 100 ATP molecules with no energy lost as heat.
Chemiosmotic Theory and the Case for Creation
The elegant molecular rationale that undergirds the use of proton gradients to harvest energy and to power certain cellular processes makes it unlikely that this biochemical feature reflects the outcome of a historically contingent process that just happened upon proton gradients. Instead, it points to a set of principles that underlie the structure and function of biochemical systems—principles that appear to have been set in place from the beginning of the universe.
The most obvious and direct way for the first protocells to harvest energy would seemingly involve some type of mechanism that resembled substrate-level phosphorylation, not an indirect and more complicated mechanism that relies on proton gradients. If the origin of chemiosmosis and the use of proton gradients was, indeed, a historically contingent outcome—predicated on the fact that the first protocells just happened to employ a natural proton gradient—it seems almost eerie to think that evolutionary processes blindly stumbled upon what would later become such an elegant and efficient energy-harvesting process. And a process necessary for advanced life to be possible on Earth.
If not for chemiosmosis, it is unlikely that eukaryotic cells and, hence, complex life such as animals, plants, and fungi, could have ever existed. Substrate-level phosphorylation just isn’t efficient enough to support the energy demands of eukaryotic organisms.
It is also difficult to imagine how the natural proton gradients exploited by the first protocells could have been co-opted and then evolved so quickly into the complex components of the electron transport chain and F1-F0ATPase coupling mechanism found in cells that preceded LUCA. Not only are the components of the electron transport chain complex, but they have to work together in an integrated manner to establish the proton gradient across mitochondrial membranes (and the plasma membranes of bacteria and archaea). Without the existence of the F1-F0ATPase (or some other mechanism) to couple proton gradients to the synthesis of ATP, the generation of proton gradients would be for naught. The origin of the electron transport chain and F1-F0ATPase have to coincide.
On the other hand, the ingenious use of proton gradients and the elegant molecular logic that accounts for their universal use by living systems are exactly the features I would expect if life stems from the work of a Mind. Moreover, the architecture and operation of complex I and F1-F0ATPase add to the case for creation. These two complexes are molecular motors that bear an startling similarity to man-made machines, revitalizing the Watchmaker argument for God’s existence.
As noted, the use of proton gradients points to a set of deep, underlying principles that arise from the very nature of the universe itself and dictate how life must be. The molecular rationale that undergirds the use of proton gradients and their near-universal occurrence in living organisms suggests that proton gradients are an indispensable feature of living organisms. In other words, without the use of proton gradients to harvest energy and drive cellular processes, advanced life would not be possible. Or another way to say it: if life was discovered elsewhere in the universe, it would have to employ proton gradients to harvest energy.
It is remarkable to think that proton gradients, which are a manifestation of the laws of nature, are, at the same time, precisely the type of system advanced life needs to exist. One way to interpret this “coincidence” is that it serves as evidence that our universe has been designed for a purpose.
And as a Christian, I find that notion to resonate powerfully with the idea that life manifests from an intelligent Agent—namely, God.
Resources
- The Cell’s Design: How Chemistry Reveals the Creator’s Artistry by Fazale Rana (Book)
- “Electron Transport Chain Protein Complexes Rev Up the Case for Design” by Fazale Rana (article)
- “Reactive Oxygen Species: Harbingers of Evolution or Signals of Design?” by Fazale Rana (article)
- “A Periodic Table for Protein Structure Reveals Biochemical Design” by Fazale Rana (article)
Check out more from Reasons to Believe @Reasons.org
Endnotes
- John Prebble, “Peter Mitchell and the Ox Phos Wars,” Trends in Biochemical Sciences 27 (April 1, 2002): 209–12, doi:10.1016/S0968-0004(02)02059-5.
- Leslie E. Orgel, “Are You Serious, Dr. Mitchell?” Nature 402 (November 4, 1999): 17, doi:10.1038/46903.
- Nick Lane, John F. Allen, and William Martin, “How Did LUCA Make a Living? Chemiosmosis in the Origin of Life,” Bioessays 32 (2010): 271–80, doi:10.1002/bires.200900131.
- Nick Lane, “Why Are Cells Powered by Proton Gradients?” Nature Education 3 (2010): 18.
- Nick Lane, “Bioenergetic Constraints on the Evolution of Complex Life,” Cold Spring Harbor Perspectives in Biology 6 (2014): a015982, doi:10.1101/cshperspect.a015982.