Membrane Biochemistry Challenges Route to Evolutionary Origin of Complex Cells
If you find yourself in need of directions when traveling through New England and you ask a local for help, he or she just might reply, “You can’t get there from here.” As you can imagine, these aren’t welcome words, particularly when all you want to do is make your way to your destination.
Unfortunately, the same thing could be said to biologists trying to discover the evolutionary route that led to the emergence of complex, eukaryotic cells. No matter the starting point, it seems as if you just can’t get there from here.
This frustration becomes most evident as evolutionary biologists try to account for the biochemical makeup of the membranes found in eukaryotic cells. In my opinion, this struggle is not just an inconvenient detour. As the following paragraphs show, obstacles line the roadway, ultimately leading to a dead end that exposes the shortcomings of the endosymbiont hypothesis—a cornerstone idea in evolutionary biology.
Endosymbiont Hypothesis
Most biologists believe that the endosymbiont hypothesis stands as the best explanation for the origin of complex cells. According to this hypothesis, complex cells originated when symbiotic relationships formed among single-celled microbes after free-living bacterial and/or archaeal cells were engulfed by a “host” microbe.
The mitochondrion represents the “poster child” of the endosymbiont hypothesis. Presumably, this organelle started as an endosymbiont. Evolutionary biologists believe that once engulfed by the host cell, the microbe took up permanent residency, growing and dividing inside the host. Over time, the endosymbiont and host became mutually interdependent, with the endosymbiont providing a metabolic benefit—such as a source of ATP—for the host cell. In turn, the host cell provided nutrients to the endosymbiont. Presumably, the endosymbiont gradually evolved into an organelle through a process referred to as genome reduction. This reduction resulted when genes from the endosymbiont’s genome were transferred into the genome of the host organism.
Evidence for the Endosymbiont Hypothesis
1. Most of the evidence for the endosymbiont hypothesis centers around mitochondria and their similarity to bacteria. Mitochondria are about the same size and shape as a typical bacterium and have a double membrane structure like gram-negative cells. These organelles also divide in a way that is reminiscent of bacterial cells.
2. Biochemical evidence also exists for the endosymbiont hypothesis. Evolutionary biologists view the presence of the diminutive mitochondrial genome as a vestige of this organelle’s evolutionary history. They see the biochemical similarities between mitochondrial and bacterial genomes as further evidence for the evolutionary origin of these organelles.
3. The presence of the unique lipid, cardiolipin, in the mitochondrial inner membrane also serves as evidence for the endosymbiont hypothesis. This important lipid component of bacterial inner membranes is not found in the membranes of eukaryotic cells—except for the inner membranes of mitochondria. In fact, biochemists consider it a signature lipid for mitochondria and a vestige of the organelle’s evolutionary history. So far, the evolutionary route looks well-paved and clear.
Discovery of Lokiarchaeota
Evolutionary biologists have also developed other lines of evidence in support of the endosymbiont hypothesis. For example, biochemists have discovered that the genetic core (DNA replication and the transcription and translation of genetic information) of eukaryotic cells resembles that of the archaea. This similarity suggests to many biologists that a microbe belonging to the archaeal domain served as the host cell that gave rise to eukaryotic cells.
Life scientists think they may have determined the identity of that archaeal host. In 2015, a large international team of collaborators reported the discovery of Lokiarchaeota, a new phylum belonging to the archaea. This phylum clusters with eukaryotes on the evolutionary tree. Analysis of the genomes of Lokiarchaeota identifies a number of genes involved in membrane-related activities, suggesting that this microbe may well have possessed the ability to engulf other microbes.1 At this point, it looks like “you can get there from here.”
Challenges to the Endosymbiont Hypothesis
Despite this seemingly compelling evidence, the evolutionary route to the first eukaryotic cells is littered with potholes. I have written several articles detailing some of the obstacles. (See Challenges to the Endosymbiont Hypothesis in the Resources section.) Also, a divide on the evolutionary roadway called the lipid divide compounds the problem for the endosymbiont hypothesis.
Lipid Divide
The lipid divide refers to the difference in the chemical composition of the cell membranes found in bacteria and archaea. Phospholipids comprise the cell membranes of both sorts of microbes. But the similarity ends there. The chemical makeup of the phospholipids is distinct in bacteria and archaea.
Bacterial phospholipids are built around a d-glycerol backbone, which has a phosphate moiety bound to the glycerol in the sn-3 position. Two fatty acids are bound to the d-glycerol backbone at the sn-1 and sn-2 positions. In water, these phospholipids assemble into bilayer structures.
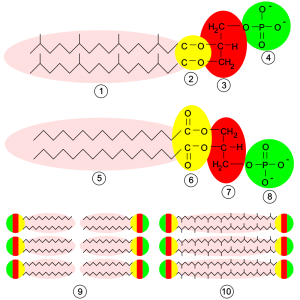
Figure: Difference between archaeal (top) and bacterial (middle and bottom) phospholipids. Features include 1: isoprene chains, 2: ether linkage, 3: l-glycerol, 4 and 8: phosphate group, 5: fatty acid chains, 6: ester linkages, 7: d-glycerol, 9: lipid bilayer of bacterial membranes, 10: lipid monolayer found in some archaea. Image credit: Wikipedia
Archaeal phospholipids are constructed around an l-glycerol backbone (which produces membrane lipids with different stereochemistry than bacterial phospholipids). The phosphate moiety is attached to the sn-1 position of glycerol. Two isoprene chains are bound to the sn-2 and sn-3 positions of l-glycerol via ether linkages. Some archaeal membranes are formed from phospholipid bilayers, while others are formed from phospholipid monolayers.
Presumably, the structural features of the archaeal phospholipids serve as an adaptation that renders them ideally suited to form stable membranes in the physically and chemically harsh environments in which many archaea find themselves.
Lipid Divide Frustrates the Origin of Eukaryotic Cell Membranes
In light of the lipid divide and the evidence that seemingly indicates that the endosymbiotic host cell likely belonged to Lokiarchaeota, it logically follows that the membrane composition of eukaryotic cells should be archaeal-like. But, this expectation is not met and the evolutionary route encounters another pothole. Instead, the cell membranes of eukaryotic cells closely resemble bacterial membranes.
One way to repair the roadway is to posit that during the evolutionary process that led to the emergence of eukaryotic cells, a transition from archaeal-like membranes to bacterial-like membranes took place. In fact, supporting evidence comes from laboratory studies demonstrating that stable bilayers can form from a mixture of bacterial and archaeal phospholipids, even though the lipids from the two sources have opposite stereochemistry.
Evolutionary biologists Purificación López-García and David Moreira question if evidence can be marshaled in support of this scenario for two reasons.2 First, mixing of phospholipids in the lab is a poor model for cell membranes that function as a “dynamic cell-environment interface.”3
Second, they question if this transition is feasible given how exquisitely optimized membrane proteins must be to fit into cell membranes. The nature of protein optimization is radically different for bacterial and archaeal membranes. Because cell membrane systems are optimized, the researchers question if an adequate driving force for this transition exists.
In other words, these two scientists express serious doubts about the biochemical viability of a transitional stage between archaeal membranes. In light of these obstacles, López-Garcíaand Moreira write, “The archaea-to-bacteria membrane shift remains the Achilles’ heel for these models [that propose an archaeal host for endosymbionts].”4
In other words, you can’t get there from here.
Can Lokiarchaeota Traverse the Lipid Divide?
In the midst of this uncertain evolutionary route, a recent study by investigators from the Netherlands seems to point the way toward the evolutionary origin of eukaryotic membranes.5 Researchers screened the Lokiarchaeota genome for enzymes that would take part in phospholipid synthesis with the hope of finding clues about how this transition may have occurred. They conclude that this group of microbes could not make l-glycerol-1-phosphate (a key metabolic intermediate in the production of archaeal phospholipids) because it lacked the enzyme glycerol-1-phosphate dehydrogenase (G1PDH). They also discovered evidence that suggests that this group of microbes could make fatty acids and chemically attach them to sugars. The researchers argue that Lokiarchaeota could make some type of hybrid phospholipid with features of both archaeal and bacterial phospholipids.
The team’s approach to understanding how evolutionary processes could bridge the lipid divide and account for the origin of eukaryotic membranes is clever and inventive, to be sure. But it is far from convincing for at least four reasons.
1. Absence of evidence is not evidence of absence, as the old saying goes. Just because the research team didn’t find the gene for G1PDH in the Lokiarchaeota genetic material doesn’t mean this microbe didn’t have the capacity to make archaeal-type phospholipids. Toward this end, it is important to note that researchers have not cultured any microbe that belongs to this group organisms. The group’s existence is inferred from metagenomic analysis, which involves isolating small fragments of DNA from the environment (in this case a hydrothermal vent system in the Atlantic Ocean, called Loki’s Castle) and stitching them together into a genome. The Lokiarchaeota “genome” is low quality (1.4-fold coverage) and incomplete (8 percent of the genome is missing). Around one-third (32 percent) of the genome codes for proteins with unknown function. Could it be that an enzyme capable of generating l-glycerol-1-phosphate exists in the mysterious third of the genome? Or in the missing 8 percent?
2. While the researchers discovered that genes could conceivably work together to make d-glycerol-3-phosphate (though the enzymes encoded by these genes perform different metabolic functions), they found no direct evidence that Lokiarchaeota produces d-glycerol-3-phosphate. Nor did they find evidence for glycerol-3-phosphate dehydrogenase (G3PDH) in the Lokiarchaeota genetic material. This enzyme plays a key role in the synthesis of phospholipids in bacteria.
3. Though the researchers found evidence that Lokiarchaeota had the capacity to make fatty acids, some of the genes required for the process seem to have been acquired by these microbes via horizontal gene transfer with genetic material from bacteria. (It should be noted that 29 percent of the Lokiarchaeota genome comes from the bacteria.) It is not clear when Lokiarchaeota acquired these genes and, therefore, if this metabolic capability has any bearing on the origin of eukaryotes.
4. The researchers present no evidence that Lokiarchaeota possessed the protein machinery that would chemically attach isoprenoid lipids to d-glycerol-3-phosphate via ether linkages.
Thus, the only way to establish Lokiarchaeota membranes as a transitional evolutionary pathway between those found in Archaea and Bacteria is to perform chemical analysis of its membranes. At this juncture, such analysis is impossible to perform because no one has been able to culture Lokiarchaeota. In fact, other evidence suggests that this group of microbes possessed archaeal-type membranes. Researchers have recovered archaeal lipids in the sediments surrounding Loki’s Castle, but they have not recovered bacterial-like lipids.
More Lipid Divide Frustration
Given these problems, could it be that the host microbe for the endosymbiont was a member of Bacteria, not Archaea? While this model would solve the problem of the lipid divide, it leaves unexplained the similarity between the genetic core of eukaryotes and the Archaea. Nor does it account for the grouping of eukaryotes with the Archaea.
It doesn’t look like you can get there from here, either.
Evolutionary biologists Jonathan Lombard, Purificación López-García and David Moreira sum things up when they write, “The origin of eukaryotic membranes is a problem that is rarely addressed by the different hypotheses that have been proposed to explain the emergence of eukaryotes.”6 Yet, until this problem is adequately addressed, the evolutionary route to eukaryotes will remain obscure and the endosymbiont hypothesis noncompelling.
In light of this challenge and others, maybe a better way to make sense of the origin of eukaryotic cells is to view them as the Creator’s handiwork. For many scientists, it is a road less traveled, but it accounts for all of the data. You can get there from here.
Resources
Challenges to the Endosymbiont Hypothesis
- “Evolutionary Paradigm Lacks Explanation for Origin of Mitochondria and Eukaryotic Cells” by Fazale Rana (article)
- “Complex Protein Biogenesis Hints at Intelligent Design” by Fazale Rana (article)
- “Endosymbiont Hypothesis: Things Aren’t What They Seem to Be” by Fazale Rana (article)
Support for a Creation Model for the Origin of Eukaryotic Cells
- “Why Do Mitochondria Have DNA?” by Fazale Rana (article)
- “Mitochondrial Genomes: Evidence for Evolution or Creation?” by Fazale Rana (article)
- “Mitochondria’s Deviant Genetic Code: Creation or Evolution?” by Fazale Rana (article)
- “Can a Creation Model Explain the Origin of Mitochondria?” by Fazale Rana (article)
- “Molecular Logic of the Electron Transport Chain Supports Creation” by Fazale Rana (article)
- “Why Mitochondria Make My List of Best Biological Designs” by Fazale Rana (article)
Check out more from Reasons to Believe @Reasons.org
Endnotes
- Anja Spang et al., “Complex Archaea that Bridge the Gap between Prokaryotes and Eukaryotes,” Nature 521 (May 14, 2015): 173–79, doi:10.1038/nature14447; Katarzyna Zaremba-Niedzwiedzka et al., “Asgard Archaea Illuminate the Origin of Eukaryotic Cellular Complexity,” Nature 541 (January 19, 2017): 353–58, doi:10.1038/nature21031.
- Purificación López-García and David Moreira, “Open Questions on the Origin of Eukaryotes,” Trends in Ecology and Evolution 30, no. 11 (November 2015): 697–708, doi:10.1016/j.tree.2015.09.005.
- López-García and Moreira, “Open Questions.”
- López-García and Moreira, “Open Questions.”
- Laura Villanueva, Stefan Schouten, and Jaap S. Sinninghe Damsté, “Phylogenomic Analysis of Lipid Biosynthetic Genes of Archaea Shed Light on the ‘Lipid Divide,’” Environmental Microbiology 19, no. 1 (January 2017): 54–69, doi:10.1111/1462-2920.13361.
- Jonathan Lombard, Purificación López-García, and David Moreira, “The Early Evolution of Lipid Membranes and the Three Domains of Life,” Nature Reviews Microbiology 10 (June 11, 2012): 507–15, doi:10.1038/nrmicro2815.





