Krebs Cycle Origin Brings Case for Creation Full Circle
My wife and I have visited London on several occasions. While there, we have always relied on the Underground and the city buses for transportation. Neither of us ever mustered up enough courage to hire (rent) a car. I won’t speak for my wife, but I have always been a bit hesitant about driving on the opposite side of the road. Yet, if I needed to, I think I could manage driving in London‘s quieter areas with a little bit of practice and a whole lot of concentration. Still, I would be hard-pressed to ever become comfortable enough to try driving into some of London’s busiest and more complex roundabouts.
The roundabouts of London serve as fitting metaphors for the biochemical cycles that contribute to cellular metabolic pathways. These cycles are complex, to say the least, and can be difficult for first-year biochemistry students to master. Yet, becoming conversant with the design of metabolic cycles is well worth the effort. This mastery is essential if biochemistry students hope to understand and navigate some of life’s most important biochemical processes.
Some life scientists think that understanding the design and origin of the Krebs cycle (one of life’s most important and central metabolic pathways) just might be key to understanding the origin of life. Collaborators from Furman University and the Scripps Research Institute recently highlighted features of the Krebs cycle that seem to support a chemical evolutionary origin of intermediary metabolism and, hence, the origin of life.1 But careful consideration of the team’s findings suggests that life’s origin more likely stems from the work of a Creator.
But before we discuss their work and its implications, a bit of background information might be helpful. (For readers familiar with intermediary metabolism and the Krebs cycle, skip ahead to “The Krebs Cycle and the Origin of Life.”)
Intermediary Metabolism
Metabolism refers to the myriad life-sustaining chemical reactions that occur in the cell (and extracellular space in multicellular organisms) that are necessary to sustain life. Metabolic activity makes it possible for life-forms to extract energy from the environment and construct life’s components. These processes allow organisms to grow, reproduce, maintain biological structures, and respond to changes in the environment. Metabolic reactions include the production and breakdown of proteins and RNA molecules, DNA replication, and the assembly of cell membranes and cell walls.
Metabolism also involves reactions of small molecules within the cell’s interior. A significant number of metabolic reactions produce small molecules used by the cell’s machinery as building blocks to assemble proteins, DNA, RNA, and cell membrane bilayers. On the other hand, some metabolic activities break down compounds like glucose and lipid molecules into smaller molecules to provide energy for the cell’s operations. Some metabolic activities prepare materials the cell no longer needs (cellular waste) for elimination. Other reactions detoxify materials that are harmful to the cell.
Within the cell’s interior, metabolic processes are often organized like city streets into routes or pathways comprised of a series of chemical reactions. These reactions transform a starting compound into a final product via a series of small, stepwise chemical changes. Each step in a metabolic route is mediated by a protein (called an enzyme) that assists in the chemical transformation. The pathways can be linear, branched, or, circular, which is the case for the Krebs cycle.
The chemical components that form part of one metabolic sequence sometimes take part in other pathways. These shared compounds cause metabolic pathways to be interconnected and networked together. The sum total of metabolic processes represents a complex, reticulated web of chemical reactions, each one catalyzed by an enzyme.
Credit: Shutterstock
The Krebs Cycle
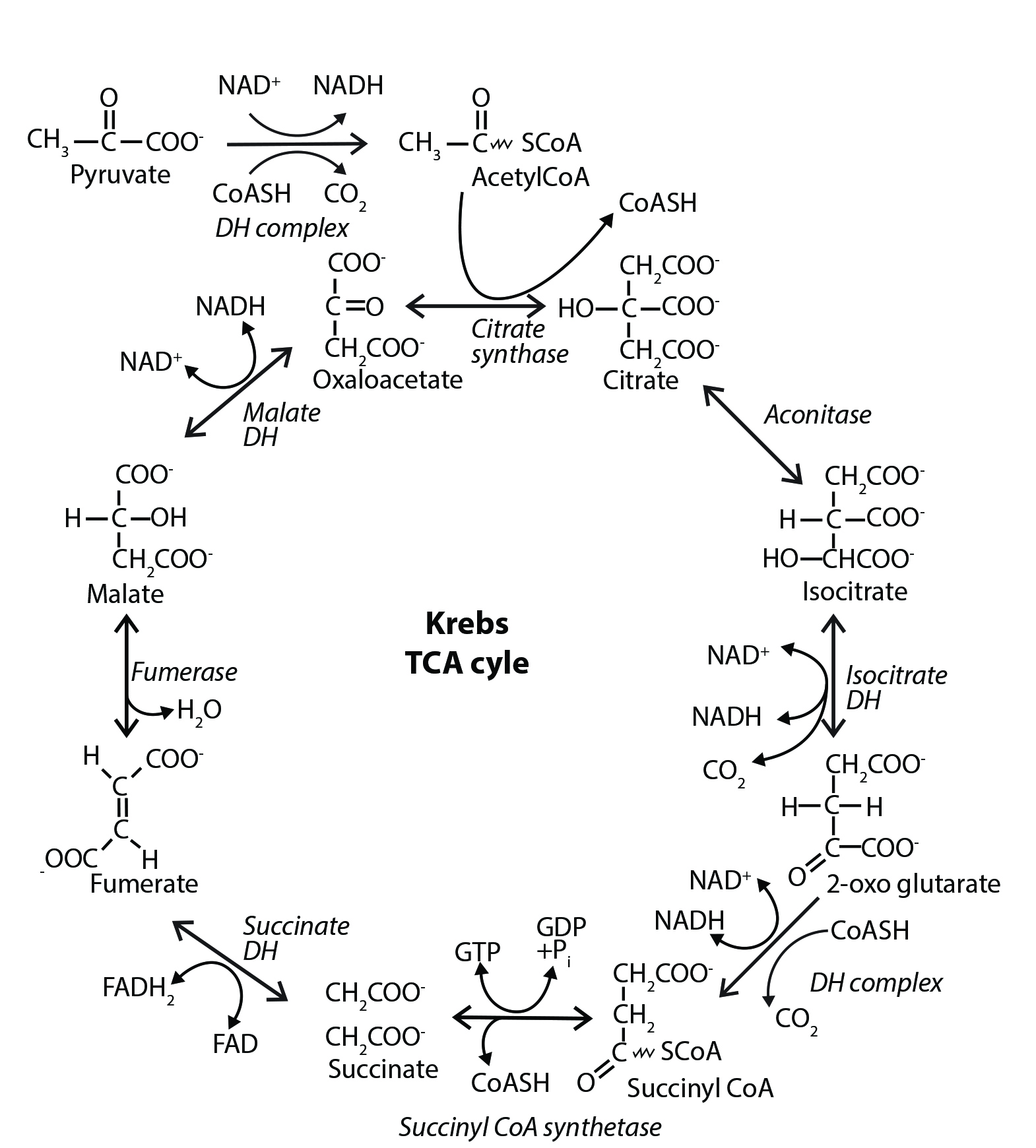
The Krebs cycle (sometimes called the tricarboxylic acid cycle or the citric acid cycle) oxidizes the pyruvate generated from the glycolytic pathway into carbon dioxide and water. This combustion process liberates a significant amount of energy which, in turn, is captured and used to drive the formation of NADH and FADH2. Once formed, the two molecules feed high-energy electrons into the electron transport chain, which uses the electrons to form a proton gradient across the mitochondrial inner membrane. The proton gradient drives the formation of ATP molecules through a process called oxidative phosphorylation. This process requires molecular oxygen and generates over 90% of the ATP used by aerobic organisms.
The Krebs cycle is also part of central carbon metabolism—with many of its intermediates serving as the precursors used to generate building block compounds such as glucose, fatty acids, amino acids, nucleobases, and the porphyrin ring (which is used, for example, as the cofactor for the hemoglobin molecule). In fact, the Krebs cycle functions as the central hub for intermediary metabolism, integrating many of the individual metabolic routes in the cell.
The Krebs Cycle and the Origin of Life
The Krebs cycle assumes a central role in intermediary metabolism and it occurs nearly universally throughout the living realm. For these reasons, many origin-of-life researchers think that this metabolic route may have been one of the first metabolic pathways to arise, predating the emergence of LUCA (the last universal common ancestor).
Interestingly, some bacteria run the Krebs cycle in reverse. (This pathway is called the reverse Krebs cycle.) Instead of converting pyruvate into carbon dioxide, water, and high-energy electrons (associated with NADH and FADH2), this pathway converts carbon dioxide and high-energy electrons harvested from minerals in the environment to generate organic materials. In fact, some origin-of-life researchers think that the reverse Krebs cycle predated the origin of primitive genetic material and primitive cell boundaries. As such, the reverse Krebs cycle operated as an autocatalytic cycle, kickstarting the process of abiogenesis. For some origin-of-life researchers, understanding the evolutionary pathway that led to the reverse Krebs cycle provides them with a vital clue as they seek to make sense of the mystery of life’s origin.
Pioneering Studies of Harold Morowitz
In 2000, Harold Morowitz and a team of collaborators sought to identify the types of prebiotic compounds that might emerge under the conditions of early Earth. They hoped that this list of compounds would help them determine if, in principle, the Krebs cycle could emerge spontaneously on early Earth.2 Morowitz and his team screened 3.5 million entries in Beilstein’s compendium of organic compounds, applying a set of rules that reflected a realistic set of physical and chemical constraints relevant to the prebiotic conditions of early Earth. Specifically, they focused on relatively low molecular weight compounds consisting of carbon, hydrogen, and oxygen that are water–soluble and possess either aldehyde or ketone functional groups. They excluded compounds with high heats of combustion, compounds that were chemically unstable, and compounds that contained functional groups that would be challenging to generate under plausible prebiotic conditions. Applying this filter, they winnowed the 3.5 million Beilstein entries to 153 compounds, which included all the Krebs cycle intermediates. For Morowitz and his team, this result meant that the compounds that form the Krebs cycle are emergent features on early Earth that flow naturally out of the properties of carbon chemistry and Earth’s early conditions. In fact, they argue that the Krebs cycle intermediates may well constitute a unique set of molecules.
Prebiotic Production of Krebs Cycle Compounds
Building on the work of Morowitz and his colleagues, a team of researchers from France reported in 2019 that they could generate nine of the eleven compounds that constitute Krebs cycle intermediates by incubating either pyruvate or glyoxylate in water under an inert atmosphere at 70°C in the presence of ferrous iron.3 This set of conditions models mild hydrothermal vent conditions on early Earth. Conceivably, pyruvate and glyoxylate could have been generated in such an environment.
In 2020, a team of investigators from Furman University and the Scripps Institute demonstrated that all the ingredients for a precursor of the Krebs cycle could be generated from glyoxylate and pyruvate in a single reaction vessel under mild temperatures and pH conditions without the need for metal catalysts.4 They discovered that the transformations proceeded in the same sequence as the sequence of intermediates in the reverse Krebs Cycle. The team also discovered that the components of the sequence could be converted into amino acids via a transamination reaction.
On the surface, this work provides validation for chemical evolution in general and, for a metabolism-first approach to the origin of life specifically. Greg Springsteen, a member of the research team notes, “Comparatively, the chemistry we discovered here was a dream to run: All you really needed to get the pathway started was [to] drop two stable reactants into buffered water and stick it on a warm hotplate. The chemistry was extremely robust.”5
Still, some measure of caution is in order. Neither of the experiments carried out by the Furman and Scripps scientists or the work of the French researchers successfully generated an autocatalytic cycle. Instead, these two experiments generated a mixture of molecules. A mechanism for transforming the mixture into a bona fide chemical cycle has yet to be discovered.
An additional damper on the success of these studies comes from previous work that has identified seemingly intractable problems with metabolism-first scenarios in general and, specifically, for the emergence of the reverse Krebs cycle under the conditions of early Earth.
Failure of Metabolism-First Models
One of the most significant critiques of metabolism-first scenarios came from the late origin-of-life researcher Leslie Orgel. (For other critiques see Challenges for Metabolism-First Scenarios in the resources section.)
Using the reverse Krebs cycle as a case study, Orgel identified several problems confronting metabolism-first scenarios that all center around their geochemical plausibility.6 For example, Orgel pointed out that one of the chief problems facing metabolism-first models is the need for highly specific mineral catalysts. As a case in point, the reverse citric acid cycle consists of eleven reaction steps, each one requiring a specific mineral catalyst on the early Earth. Inside cells, this metabolic process employs complex enzyme catalysts possessing high specificities and capacities for molecular-level discrimination among the components of the cycle. But enzymes would not have been available on early Earth. Orgel argues that it’s not likely that the right types of minerals needed to carry out these reactions would coexist at a particular locale on the early Earth in such a way to support the reverse citric acid cycle.
Adding to this problem is the observation that the transition metal catalysts (associated with the mineral surfaces) not only facilitate chemical transformations but also prompt the decomposition of the proto-metabolic intermediates. As biochemist Juli Pereto states, “Metals and harsh conditions can be good [at] accelerating the reactions yet also [promote] the destruction of the products. This situation makes rather implausible or unrealistic some of the proposed schemes.”7
In some respects, the Furman and Scripps researchers‘ work may address these two concerns. They were able to generate the Krebs cycle intermediates under mild conditions without the use of metal catalysts.
Still, other concerns remain. Orgel also pointed out that the autocatalytic chemical cycles that would be necessary to support metabolism-first scenarios would be highly susceptible to chemical disruption on early Earth. Earth’s chemical environment would have been a chemically complex setting, to say the least. The intermediates of the reverse Krebs cycle would not only react with one another, but also with other materials present. These side reactions would disrupt the proto-metabolic cycle. In other instances, the side reactions would stultify the autocatalytic cycle by siphoning off its intermediates.
So, even though the scientists from Furman University and the Scripps Research Institute have demonstrated, in principle, a chemical process that could contribute to the emergence of the Krebs cycle (and, hence, the origin of life), they have a long way to go to demonstrate geochemical relevance of their work.
In the face of these (and other) significant challenges to metabolism-first scenarios—that, at the end of the day, may well be intractable—it is important not to overlook something rather provocative that arises out of studies designed to understand the evolutionary origin of the Krebs cycle. For, even if we concede that life did, indeed, have an evolutionary origin, it is impossible to escape the necessary role a Creator must have played in the appearance of first life on Earth.
Anthropic Coincidences and the Evolutionary Origin of the Krebs Cycle
Based on the research discussed here, it doesn’t appear as if the Krebs cycle is the outworking of a historically contingent evolutionary process. Instead, if the Krebs cycle evolved, it appears as if this pathway arose out of the dictates of carbon chemistry and the geochemical conditions of early Earth. As origin-of-life researchers Eric Smith and Harold Morowitz argue:
“The chart of intermediary metabolism has a universal anabolic core, which should not be understood as merely a result of common ancestry but rather as a solution imposed on early life within the energetically structured environment of the early earth by details of carbon chemistry and carbon transportation functions performed only by biomass.”8
As I discuss in detail in my upcoming book Fit for a Purpose (to be released in the fall of 2021), the design of the Krebs cycle and the physicochemical properties of its intermediates are just right. They make it possible for this metabolic pathway to serve its dual roles in harvesting energy to power the cell’s operation and operating as the hub in central carbon metabolism. In other words, the Krebs cycle’s constitution and design appear to be ideally suited for life.
It is equally remarkable to think that both pyruvate and glyoxylate, which could have conceivably formed at hydrothermal vents, have the just-right set of chemical and physical properties that allow these two compounds to generate Krebs cycle intermediates. For example, both are stable in water and have a propensity to form carbon-carbon bonds. Additionally, the Furman and Scripps team discovered that glyoxylate not only functions as a reactant, but also as a reducing agent that facilitates the production of the Krebs cycle constituents. To put it another way, glyoxylate has a just-right set of properties that make this prebiotic reaction sequence possible.
In other words, there appear to be constraints on prebiotic chemistry that inevitably lead to the production of key biotic molecules with the just-right properties that make them unusually stable and ideally suited to give rise to one of the most important metabolic pathways in living systems.
It is a bit eerie to think that (1) the constraints that arise out of the geochemical and geophysical settings of the early Earth, (2) the physicochemical constraints that arise out of carbon chemistry, and (3) the interplay between organic compounds and water all conspire to spontaneously generate the compounds which serve as metabolic intermediates for the Krebs cycle.
This remarkable set of coincidences is highly fortuitous, suggesting a fitness for purpose to the nature of prebiotic chemistry. There is an apparent teleology to prebiotic chemistry. The laws of physics and chemistry may well have been rigged at the onset to ensure that life’s building blocks emerge naturally under early Earth’s conditions.
Could it be that these coincidences reflect the fact that a Creator is behind it all?
Resources
Origins of Life by Fazale Rana and Hugh Ross (book)
Creating Life in the Lab by Fazale Rana (book)
Questioning the Geochemical Relevance of Prebiotic Chemistry
“Prebiotic Chemistry and the Hand of God” by Fazale Rana (article)
Challenges for Metabolism-First Scenarios
“A Fork in the Road, Part 2” by Fazale Rana (article)
“Grave Concerns about Metabolism-First Origin-of-Life Scenarios” by Fazale Rana (article)
“Metabolism-First Models Can’t Evolve” by Fazale Rana (article)
Prebiotic Chemistry and the Anthropic Principle
“Have Researchers Developed a Computer Algorithm that Explains the Origin of Life?” by Fazale Rana (article)
The Biochemical Anthropic Principle
“A New Mechanism for Movement of Biochemical Cargo Orders the Case for a Creator” by Fazale Rana (article)
“Molecular Logic of the Electron Transport Chain Supports Creation” by Fazale Rana (article)
“The Logic of DNA Replication Makes a Case for Intelligent Design” by Fazale Rana (article)
“A Periodic Table for Protein Structures Reveals Biochemical Design” by Fazale Rana (article)
“Biochemical Grammar Communicates the Case for Creation” by Fazale Rana (article)
“Is the Optimal Set of Protein Amino Acids Purposed by a Mind?” by Fazale Rana (article)
“Fatty Acids Are Beautiful” by Fazale Rana (article)
Check out more from Reasons to Believe @Reasons.org
Endnotes
1. Trent R. Stubbs et al., “A Plausible Metal-Free Ancestral Analogue of the Krebs Cycle Composed Entirely of alpha-Ketoacids” Nature Chemistry 12 (October 12, 2020): 1016–1022, doi: 10.1038/s41557-020-00560-7.
2. Harold J. Morowitz et al., “The Origin of Intermediary Metabolism,” Proceedings of the National Academy of Sciences, USA 97, no. 14 (July 5, 2000): 7704–7708, doi: 10.1073/pnas.110153997.
3. Kamila B. Muchowska, Sreejith J. Varma and Joseph Moran, “Synthesis and Breakdown of Universal Metabolic Precursors Promoted by Iron” Nature 569 (2019): 104–107; doi: 10.1038/s41586-019-1151-1; Robert Pascal, “A Possible Non-Biological Reaction Framework for Metabolic Processes on Early Earth,” Nature 569 (2019): 47–49, doi: 10.1038/d41586-019-01322-3
4. Stubbs et al., “Plausible Metal-Free Ancestral Analogue,” 1016–1022.
5. Glen Harvey, “New Clues to the Chemical Origin of Metabolism at the Dawn of Life,” Quanta Magazine (October 12, 2020), quantamagazine.org/new-clues-to-chemical-origins-of-metabolism-at-dawn-of-life-20201012/.
6. Leslie Orgel, “The Implausibility of Metabolic Cycles on Prebiotic Earth,” PLoS Biology 6 (January 22, 2008): e18; doi: 10.1371/journal.pbio.0060018.
7. Harvey, “New Clues,” quantamagazine.org/new-clues-to-chemical-origins-of-metabolism-at-dawn-of-life-20201012/.
8. Eric Smith and Harold J. Morowitz, “Universality in Intermediary Metabolism,” Proceedings of the National Academy of Sciences, USA 101, no. 36 (September 7, 2004): 13168–13173; doi: 10.1073/pnas.0404922101.






