A Christian Perspective on Synthetic Embryos
I carry around a lot of valuable life lessons that my high school driver’s ed teacher, Mr. Cunningham, taught us. One is: “Make sure you don’t overdrive your headlights.” It’s great advice when driving at night. In fact, it might keep you from being involved in a serious accident. If you drive so fast that the car’s stopping distance is greater than the distance illuminated by the car’s headlights, you run the risk of crashing into something you don’t even realize is there.
Making sure that you “don’t overdrive your headlights” is also a great life principle.
As much as possible, all of us want to make sure that we can anticipate what’s ahead in the future to avoid an unexpected catastrophe. Often, the only way we can see and anticipate what awaits us is to slow down and take the time to carefully think through our current circumstances and work through possible outcomes. This exercise helps prepare us for what will come.
Danger Ahead
Unfortunately, when it comes to emerging biotechnologies, scientific and technological advances are taking place so rapidly that bioethicists and social institutions have little opportunity to understand and evaluate their safety and ethical and social implications before the next set of breakthroughs take place. Each set of advances carries its own unique ethical and social challenges. Because the ethical and social implications of earlier advances haven’t been fully engaged, we run the risk of speeding into unethical territory.
It sure seems to me that we are dangerously close to “overdriving our headlights” when it comes to the creation of synthetic embryos. Recent headlines announced that a team from the Chinese Academy of Sciences produced the first-ever synthetic monkey embryos in a lab without using sperm and egg cells.1 For many people, this advance was seen as a key stepping stone toward the creation of synthetic human embryos. In fact, within a few weeks of this advance, a collaborative team from Cambridge University and Caltech announced the creation of the first human synthetic embryos at the June 2023 Meeting of the International Society for Stem Cell Research in Boston.2 This team made preprints describing their work available before publication. And within days, three separate teams from the US, China, and Israel also published preprints describing similar independent achievements.
The creation of synthetic embryos—whether animal or human—raises all sorts of ethical questions about the moral status of these cell cultures. These questions are extremely challenging to address for bioethicists and laypeople alike. The answers aren’t obvious—regardless of worldview considerations.
- How should Christians respond, generally, to advances in biotechnology?
- How should Christians respond, specifically, to the creation of synthetic embryos?
I’ll address these two questions later, but first I need to answer several basic questions about synthetic embryos:
- What are synthetic embryos?
- What have biotechnologists accomplished to date?
- Why do scientists want to create synthetic embryos?
And to properly address these questions, it is helpful to understand a bit about embryonic development. (Those readers who have this understanding, please feel free to skip ahead to What Are Synthetic Embryos?)
An Overview of Embryo Growth and Development
Embryological development begins the moment the egg cell (oocyte) becomes fertilized by a sperm cell, yielding a zygote. In turn, the zygote undergoes several rounds of cell division (referred to as cleavage) to produce a berry-like structure, called a morula. All of this happens by the third or fourth day of pregnancy.
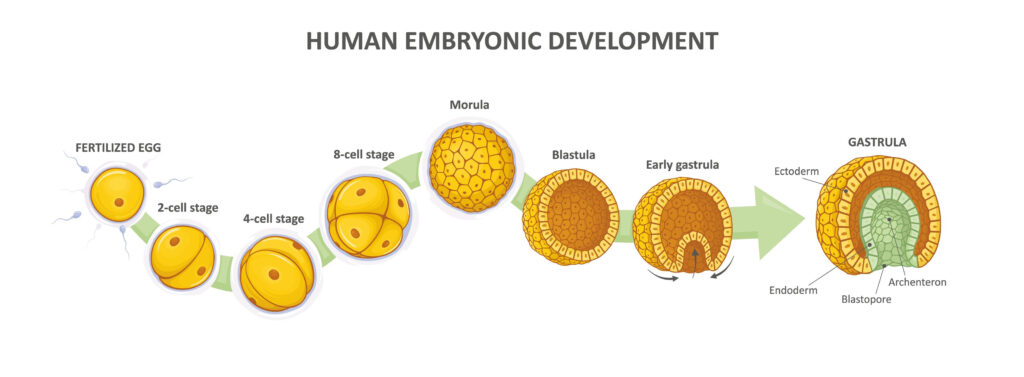
Figure 1: Embryogenesis
Credit: Shutterstock
Over the next couple of days, the morula undergoes changes that characterize the process of embryogenesis. (See figure 1.) In addition to undergoing growth and division, cells in the morula begin to migrate relative to one another to form a structure with a hollow sphere called a blastula. Within the sphere lies a clump of cells called the inner cell mass. (See figure 2.)
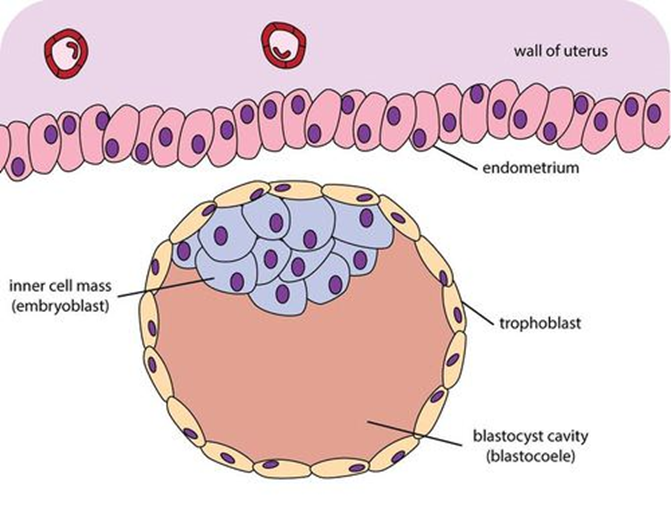
Figure 2: Inner Cell Mass
Credit: Wikipedia, https://en.wikipedia.org/wiki/Inner_cell_mass#/media/File:Diagram_of_Blastocyst_stage.png
In the next stage of embryogenesis, the inner cell mass transforms into a stack of three cellular layers (called germ layers) through cell growth, division, and migration. At this stage, the embryo is referred to as the gastrula.
The specific cell layers of the gastrula are called the ectoderm, mesoderm, and endoderm. Each of these cell layers is fated to develop into different organ systems in the body. The ectoderm forms the nervous system and the epidermis of the skin. The mesoderm forms muscles, the skeletal system, blood and blood vessels, and the dermis of the skin. The endoderm forms the linings of the digestive and respiratory systems and organs that comprise the digestive system, such as the liver and pancreas.
After gastrulation, the next stage involves organ formation. Called organogenesis, this phase begins in each of the individual cell layers and involves the careful orchestration of several processes, including cell growth, cell division, cell-to-cell communication, cell migration, differentiation of cells into specialized types, secretion of extracellular materials, and even cell death (which is necessary to sculpt the tissues and organs).
What Are Synthetic Embryos?
Synthetic embryos are highly differentiated cell cultures grown in the lab. They’re designed to be facsimiles of embryos. Instead of being formed starting with sperm and egg cells, as is the case for in vitro fertilization, synthetic embryos are cultured directly from stem cells, thereby eliminating the need for gametes. Life scientists and biotechnologists sometimes refer to synthetic embryos as blastoids or as stem-cell-based embryo models.
Generally, the stem cell type used to form synthetic embryos is derived from the blastomeres of the inner cell mass of early-stage embryos formed from in vitro fertilization. These cells are often referred to as embryonic stem cells. In principle, induced pluripotent stem cells—formed from adult fibroblast cells—could also be used to grow synthetic embryos. Alternatively, a mixture of cells taken from the inner cell mass, the trophoblast (the cell layer that becomes the placenta), and the yolk sac appear to be necessary to form synthetic mouse embryos that can progress to form rudimentary organs. (See figure 3.)
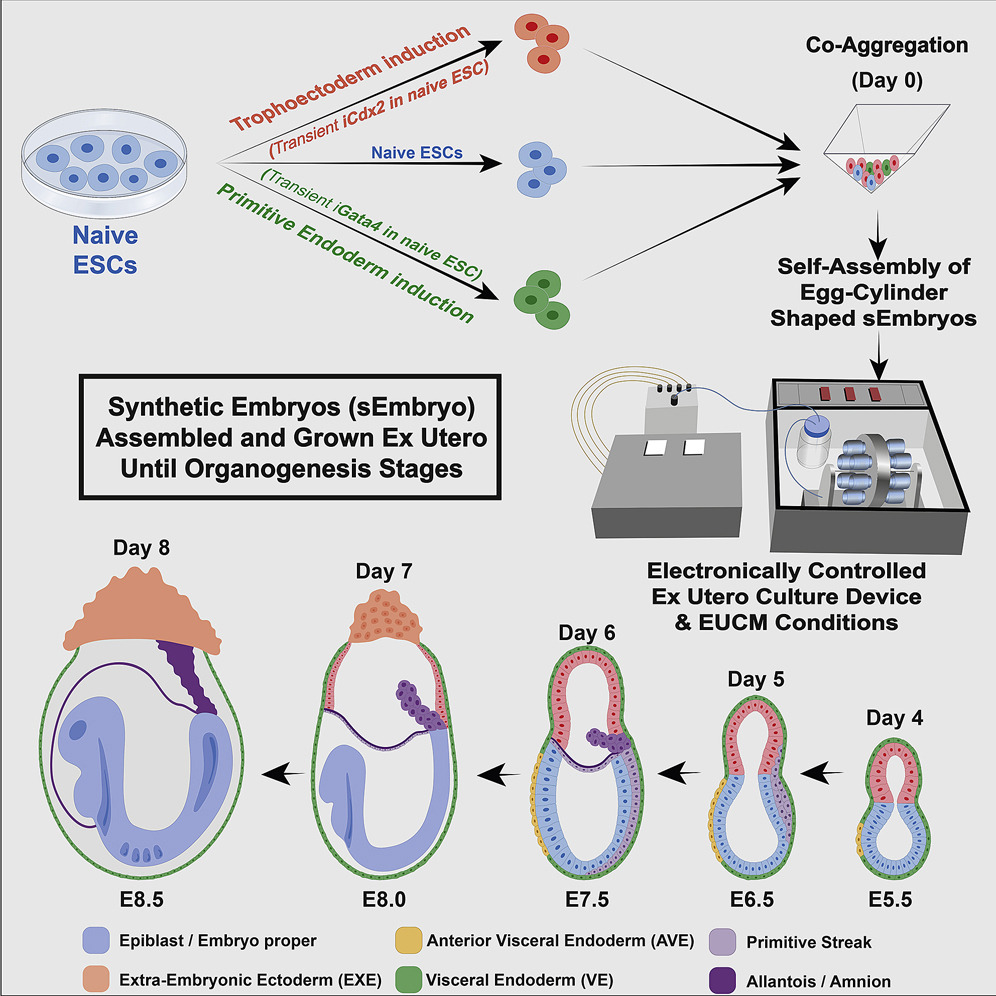
Figure 3: The Production of Synthetic Embryos
Credit: Wikipedia, https://en.wikipedia.org/wiki/Ectogenesis#/media/File:Post-gastrulation_synthetic_embryos_generated_ex_utero_from_mouse_naive_ESCs.jpg
What Have Biotechnologists Accomplished to Date?
Biotechnologists have been trying to create synthetic embryos from stem cells for about two decades. One of the most significant advances toward this goal came in 2022 when independent teams—who were both competitors and collaborators—from the University of Cambridge and the Weizmann Institute of Science, respectively, created synthetic mouse embryos in laboratory cultures that developed beyond gastrulation to the stage that rudimentary organs developed, including the brain and heart.3
Of the 10,000 embryos these teams created, 50 progressed to the 8-day stage before they died. This accomplishment is significant because mice gestation is about 20 days. In other words, these synthetic embryos successfully completed one-third of the “pregnancy.” As researchers perfect the cell culture techniques used to grow synthetic embryos, the number of embryos that successfully progress to the 8-day stage (and beyond) is expected to rise.
This work was followed up less than a year later by an advance reported by a team from the Chinese Academy of Sciences. These investigators created synthetic monkey embryos starting with embryonic stem cells. Part of the motivation for this work was to produce model synthetic embryos that more closely resembled humans (both monkeys and humans are primates) than do mice blastoids.4
The team demonstrated that the synthetic monkey embryos could develop into structures that closely resembled natural monkey embryos (produced by in vitro fertilization using sperm and egg cells) all the way through gastrulation. They also showed that blastomeres isolated from the synthetic embryos behaved in the same way in cell cultures as blastomeres isolated from natural monkey embryos. Finally, they showed that the gene expression profiles of the synthetic monkey embryos matched natural embryos.
They then implanted the synthetic embryos into the womb of a surrogate monkey and observed that the synthetic embryos implanted in the uterine wall, forming a gestational sac. After 20 days, the gestational sac was absorbed by the surrogate and disappeared altogether.
Between the summer of 2020 and the end of 2021, three independent research teams demonstrated that they could create blastula-like structures from either human embryonic stem cells or human-induced pluripotent stem cells.5 A more recent advance by four independent teams from the US, the UK, Israel, and China extends this earlier work. These four research teams have developed more robust methods for generating synthetic embryos, allowing the synthetic human embryos to consistently persist for 14 days, well into the post-implantation stage (6 days).6
These achievements are harbingers of things to come. The research teams generating synthetic human embryos are comprised of some of the best scientists and biotechnologists in the world. Based on what we have witnessed in the past few years, I don’t think it will be long before these teams continue to achieve new milestones with synthetic embryos, extending their viability in culture and creating facsimiles of natural embryos that are more and more like the real thing.
Why Do Scientists Want to Create Synthetic Embryos?
Broadly speaking, the scientific motivations behind the creation of both animal and human synthetic embryos are noble and well-intentioned.
The biological events that take place during the earliest stages of human embryonic growth and development are largely unknown. Jacob Hanna, one of the scientists working in this field, says that “this period is truly a black box.”7 These events can’t be studied in utero, or in vitro. It is very difficult to observe embryos in utero at this early stage of embryo growth when they first implant in the uterine wall. Human embryos created by in vitro fertilization in the lab can’t be studied beyond two weeks. Beyond this point, human embryos will die. The techniques to keep them alive haven’t been fully developed. One reason for this lack of know-how has to do with regulations that are in place in many countries. Governmental restrictions prevent human embryos from being grown beyond 14 days, at which point the embryos must be destroyed, frozen, or implanted.
There are also ethical concerns about experimenting with human embryos created by in vitro fertilization. Many scientists and biomedical researchers experience ethical disquiet about creating human embryos for the sole purpose of scientifically investigating them. Compounding this problem, creating embryos through in vitro fertilization requires a source of eggs, which must come from female donors. This necessity raises concerns about the exploitation of women, particularly those who are socioeconomically disadvantaged.
Many scientists and biomedical researchers don’t regard synthetic embryos as bona fide embryos. So, in their minds, they can sidestep government restrictions and ethical qualms by studying synthetic embryos instead of natural embryos.
Researchers hope that these investigations will:
- Provide them with laboratory models that allow them to study the early stages of embryonic development, opening what is currently a black box.
- Give them the opportunity to explore the effects of genetic mutations on embryonic development. The stem cells used to grow synthetic embryos can be genetically altered before they’re used to generate synthetic embryos.
- Help them study the effects of drugs and other chemical agents on embryonic development, helping researchers to identify teratogens (reproductive toxins).
They think that this collective insight will help biomedical researchers and practitioners prevent pregnancy loss and develop therapies to help couples struggling with infertility.
Scientists and biotechnologists also hope that synthetic embryos can be used to grow organs for transplants. A dream of biotechnologists and biomedical researchers is to use embryonic and adult stem cells to grow organs. To say the least, this will be a complex undertaking. The processes of blastulation, gastrulation, and organogenesis are poorly understood. Without this understanding, growing organs in vitro remains a challenge. Organ growth from stem cells requires manipulating the growth media and adding the right nutrients and growth factors at the right time. It also requires the use of culture techniques to coax the organs into the right three-dimensional architecture. At this juncture, biotechnologists have had little success growing organs from stem cells, despite their valiant efforts over the last couple of decades.
For this reason, researchers hope to gain the crucial understanding they need by studying embryogenesis and organogenesis in synthetic embryos. Some scientists think that synthetic embryos could even be used to grow organs directly, obviating the need to develop separate sets of cell culture techniques for each type of organ. Some bioecologists think that organs produced by synthetic embryos would be of better quality than ones grown directly through cell culture techniques because they would develop and grow in a more “natural” setting.
Of course, natural embryos produced by in vitro fertilization could be used to provide organs for transplants. Yet, apart from the obvious ethical unease, this proposal has technical limitations. This approach isn’t readily scalable for large-scale organ production. Each natural embryo produced by in vitro fertilization would require a human egg cell that must come from a human donor. It’s hard to imagine human donors being able to supply the eggs needed for large-scale organ production. Also, each natural embryo would be genetically distinct, leading to variability in the organs destined for use in transplant procedures.
Because synthetic embryos are generated from cultures of embryonic stem cells—which are “immortal” cell lines, meaning that they are capable of self-renewal, indefinitely—their production is scalable. The quality and characteristics of the resulting embryos would be reproducible because all the synthetic embryos come from the same genetically identical stem cells. The ability to reproduce genetically identical synthetic embryos also adds to their usefulness as a scientific model.
Ethical Concerns
Synthetic embryos can potentially be used for good and the scientists and biotechnologists exploring their generation operate with principled motives. Yet, the creation of embryos for research and clinical purposes is fraught with troubling ethical misgivings—at least for human synthetic embryos.
Embryos’ Limited Usefulness
Apart from outright animal cruelty, the creation and study of animal embryos is generally regarded as unproblematic, regardless of one’s worldview. While recognizing their limitations for generating understanding about human embryonic growth and development (because they are animal models), mouse and macaque synthetic embryos, for example, will advance our knowledge and may be useful in identifying the genetic basis of embryonic development and the effect of mutations and chemicals on embryo biology. But, because they aren’t human embryos, their utility for gaining insight into human embryonic development has its limitations.
At first glance, the creation of human synthetic embryos seems to solve the problem. They are human cell cultures and, consequently, more relevant than animal models. And they aren’t true embryos, just facsimiles of them.
But herein lies a problem. Because they are facsimiles of human embryos, some investigators have questioned their relevance for understanding the growth and development of natural human embryos. The question is whether any insight developed by studying synthetic human embryos applies only to the synthetic embryo models or has broader relevancy to natural human embryos.
To remedy this problem, biotechnologists and biomedical researchers are already trying to narrow the gap between the synthetic embryos they generate and natural ones. This work is necessary to make human and animal synthetic embryos truly useful as lab models and indispensable if human synthetic embryos will ever be used to grow organs for transplants.
Moral Status of Embryos
But as synthetic and natural human embryos become indistinguishable, this resemblance raises questions about the moral status of synthetic embryos. If no substantial difference exists between synthetic and natural embryos, shouldn’t the same ethical and moral considerations apply to them equally? Because of this inevitable similarity, a growing number of bioethicists are arguing that synthetic human embryos should be regarded as true human embryos, even if they are still crude facsimiles of natural ones.
Using Embryos for Reproduction
What about the use of synthetic embryos for reproductive purposes? Right now, the scientists and biotechnologists working with synthetic embryos say that they have no intention of using them for reproductive therapies. In fact, the International Society for Stem Cell Research has issued statements calling for regulations that prohibit implanting human synthetic embryos into the womb of a surrogate. (It should be noted that this scientific society has no power to enforce these recommendations.)
Today, it’s easy to abide by this restriction because synthetic embryos remain a crude facsimile of natural ones. But what happens when synthetic embryos become indistinguishable from natural embryos? The temptation will be unavoidable. Researchers will want to implant these embryos into the wombs of surrogates to assess their similarity to natural embryos and to learn about the early stages of implantation and embryo growth and development in a natural environment. As discussed earlier, Chinese scientists have done these very experiments on macaques for these very reasons. It’s easy to see how these types of experiments, though they violate the proposed guidelines, would be justified for the sake of accruing scientific knowledge. But once the techniques are worked out to perform these experiments, it’s just a few small steps away from using human synthetic embryos for reproductive purposes.
Destruction of Human Embryos
I have one final ethical concern. To create synthetic human embryos, embryonic stem cells are required. And, ultimately, the source of embryonic stem cells is human embryos generated through in vitro fertilization. In other words, the creation of synthetic human embryos requires, first, the creation of natural embryos that must be destroyed to isolate embryonic stem cells. These cells, in turn, are used to create the embryonic stem cell cultures that comprise the source of stem cells for the generation of synthetic embryos. While the number of natural human embryos created by in vitro fertilization to establish the embryonic stem cell lines is limited, human embryos still must be created and destroyed to create human synthetic embryos.
This necessity is deeply troubling for people who hold a pro-life position. One encouraging advance is the use of induced pluripotent stem cells to create human synthetic embryos, which may obviate the need for embryonic stem cells to generate these human embryo models.
So, are we overdriving our headlights? The moral status of human synthetic embryos is unclear. Nobody can confidently say what they are and what they are not. But what is clear is that we’ve put ourselves in an ethical quagmire and we may not have a way to free ourselves. As important as the creation of human synthetic embryos may be scientifically and biomedically, any reasonable person has to wonder if wisdom dictates that this work be suspended until we have time to fully work through its ethical and social implications. We also need the courage to stop this line of investigation if this is, indeed, where the ethical deliberations take us.
As physicist and writer Alan Lightman has cautioned,
I have no opposition at all to technology. I think technology is a wonderful thing that has to be used thoughtfully, and we can’t just assume that every bit of new technology improves the quality of life; it’s really in how the technology is used. What I am very disturbed about is this trend of everything happening faster and faster and faster and there being more and more general noise in the world, and less and less time for quiet reflection on who we are, and where we’re going.
Theological Mandate: Proceed with Caution
For Christians, our concern about synthetic embryos extends beyond ethical considerations. We have theological ones, as well. And these theological considerations lead to the question: Is there any biblical or theological justification for creating synthetic embryos?
I think so, but it’s a bit complex and nuanced.
The passage of Scripture most germane to this question is the account of humanity’s creation in Genesis 1. This text teaches that human beings are uniquely made in God’s image. With this special status comes certain responsibilities, including:
- Subduing the world and bringing it under our control
- Ruling over the world by exercising dominion over the creation
- Serving as caretakers of the planet
God granted dominion over creation to humans. We are to use it and the abundance of its resources so that we can flourish as human beings. Yet human flourishing should not come at the cost of a wanton disregard for the world in which we live. God charges us to exercise care for the planet and the life that inhabits it. These mandates provide the motivation for science and technology. Fulfilling each of these responsibilities requires that humanity understands the world and uses that insight to develop technologies that allow humanity to thrive, while ensuring the planet’s health and protecting Earth’s ecosystems.
Because we have dominion over creation, we are free to engage in biotechnology and synthetic biology. This freedom includes the creation of synthetic animal embryos, with an eye toward using them to foster an understanding of embryonic development that can be used to benefit humanity.
But the extent of our dominion is limited. It doesn’t extend to humans. Dominion over those creatures who bear his image belongs exclusively to God.
If we take the limitation of our dominion seriously, we should proceed cautiously with the creation of synthetic human embryos. Those made with human embryonic stem cells shouldn’t be created at all. Those made with induced pluripotent stem cells should be generated in such a way that they remain distinct from natural human embryos—if they are to be made at all.
As Mr. Cunningham taught us all those years ago: Slow down. Take your time. Make sure you know where you’re going. Make sure you can see what’s ahead of you.
Resources
Should We Play God?
- A Theology for Synthetic Biology, Part 1 by Fazale Rana (article)
- A Theology for Synthetic Biology, Part 2 by Fazale Rana (article)
Stem Cell Biology
- A New Direction for Stem Cell Research by Fazale Rana (article)
- Bringing Stem Cell Research into Focus by Fazale Rana (article)
- Embryonic-Like Stem Cells from Adult Cells by Fazale Rana (article)
- Embryonic Stem Cell Research: An Interview with Dr. Fazale “Fuz” Rana by Fazale Rana (article)
Check out more from Reasons to Believe @Reasons.org
Endnotes
- For example, see Jessica Hamzelou, “Synthetic Embryos Have Been Implanted into Monkey Wombs,” MIT Technology Review (April 6, 2023).
- Hannah Devlin, “Synthetic Human Embryos Created in Groundbreaking Advance,” The Guardian (June 14, 2023).
- Alejandro Aguilera-Castrejon et al., “Ex Utero Mouse Embryogenesis from Pre-Gastrulation to Late Organogenesis,” Nature 593 (May 6, 2021): 119–124, doi:10.1038/s41586-021-03416-3; Shadi Tarazi et al., “Post-Gastrulation Synthetic Embryos Generated Ex Utero from Mouse Naïve ESCs, Cell 185, no. 18 (September 1, 2022): 3290–3306, doi:10.1016/j.cell.2022.07.028.
- Jie Li et al., “Cynomolgus Monkey Embryo Model Captures Gastrulation and Early Pregnancy,” Cell Stem Cell 30, no. 4 (April 6, 2023): 362–377, doi:10.1016/j.stem.2023.03.009.
- Xiaodong Liu et al., “Modelling Human Blastocysts by Reprogramming Fibroblasts into iBlastoids,” Nature 591 (March 25, 2021): 627–632, doi:10.1038/s41586-021-03372-y; Leqian Yu et al., “Blastocyst-Like Structures Generated from Human Pluripotent Stem Cells,” Nature 591 (March 25, 2021): 620–626; doi:10.1038/s41586-021-03356-y; Harunobu Kagawa et al., “Human Blastoids Model Blastocyst Development and Implantation,” Nature601 (January 27, 2022): 600–605, doi:10.1038/s41586-021-04267-8.





